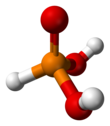
Phosphorous acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
phosphonic acid
| |||
| Other names
Dihydroxyphosphine oxide
Dihydroxy(oxo)-λ5-phosphane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.682 | ||
| KEGG | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H3PO3 | |||
| Molar mass | 82.00 g/mol | ||
| Appearance | white solid deliquescent | ||
| Density | 1.651 g/cm3 (21 °C) | ||
| Melting point | 73.6 °C (164.5 °F; 346.8 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) (decomposes) | ||
| 310 g/100 mL | |||
| Solubility | soluble in alcohol | ||
| Acidity (pKa) | 1.3, 6.7 | ||
| Structure | |||
| tetrahedral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
skin irritant | ||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | http://www.sigmaaldrich.com/MSDS/[1] | ||
| Related compounds | |||
Related compounds
|
H3PO4 (i.e., PO(OH)3) H3PO2 (i.e., H2PO(OH)) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds.
Nomenclature and tautomerism

H3PO3 is more clearly described with the structural formula HPO(OH)2. This species exists in equilibrium with a minor tautomer P(OH)3. IUPAC recommendations, 2005, are that the latter be called phosphorous acid, whereas the dihydroxy form is called phosphonic acid.[2] Only the reduced phosphorus compounds are spelled with an "ous" ending.
The P(OH)3 tautomer has been observed as a ligand bonded to molybdenum.[3][4] Other important oxyacids of phosphorus are phosphoric acid (H3PO4) and hypophosphorous acid (H3PO2). The reduced phosphorus acids are subject to similar tautomerism involving shifts of H between O and P.
Structure and oxidation state
In the solid state, HP(O)(OH)2 is tetrahedral with one shorter P=O bond of 148 pm and two longer P–O(H) bonds of 154 pm. The central phosphorus atom is assigned an oxidation state of +3.
Preparation
HPO(OH)2 is the product of the hydrolysis of its acid anhydride:
- P4O6 + 6 H2O → 4 HPO(OH)2
(An analogous relationship connects H3PO4 and P4O10).
On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam:
- PCl3 + 3 H2O → HPO(OH)2 + 3 HCl
Potassium phosphite is also a convenient precursor to phosphorous acid:
- K2HPO3 + 2 HCl → 2 KCl + H3PO3
In practice aqueous potassium phosphite is treated with excess hydrochloric acid. By concentrating the solution and precipitations with alcohols, the pure acid can be separated from the salt.
Reactions
Acid–base properties
Phosphorous acid is a strong acid with a pKa in the range 1.26–1.3.[5][6]
- HP(O)(OH)2 → HP(O)2(OH)− + H+ pKa = 1.3
It is a diprotic acid, the hydrogenphosphite ion, HP(O)2(OH)− is a moderately strong acid:
- HP(O)2(OH)− → HPO32− + H+ pKa = 6.7
The conjugate base HP(O)2(OH)− is called hydrogen phosphite, and the second conjugate base, HPO2−
3, is the phosphite ion.[7] (Note that the IUPAC recommendations are hydrogen phosphonate and phosphonate respectively).
The hydrogen bonded directly to the phosphorus atom is not readily ionizable. Chemistry examinations often test students' appreciation of the fact that all three hydrogen atoms are not acidic under aqueous conditions, in contrast with H3PO4.
Redox
Both phosphorous acid and its deprotonated forms are good reducing agents, although not necessarily quick to react. They are oxidized to phosphoric acid or its salts. It reduces solutions of noble metal cations to the metals. When phosphorous acid is treated with a cold solution of mercuric chloride, a white precipitate of mercurous chloride forms:
- H3PO3 + 2 HgCl2 + H2O → Hg2Cl2 + H3PO4 + 2 HCl
Mercurous chloride is reduced further by phosphorous acid to mercury on heating or on standing:
- H3PO3 + Hg2Cl2 + H2O → 2 Hg + H3PO4 + 2 HCl
Phosphorous acid on heating at 200 °C converts to phosphoric acid and phosphine:[8]
- 4 H3PO3 → 3 H3PO4 + PH3
Uses
The most important use of phosphorous acid (phosphonic acid) is the production of phosphites (phosphonates) which are used in water treatment. Phosphorous acid is also used for preparing phosphite salts, such as potassium phosphite. These salts, as well as aqueous solutions of pure phosphorous acid, are fungicides. Phosphites have shown effectiveness in controlling a variety of plant diseases, in particular, treatment using either trunk injection or foliar containing phosphorous acid salts is indicated in response to infections by phytophthora and pythium-type plant pathogens (both within class oomycetes, known as water molds), such as dieback/root rot and downy mildew.[9] Anti-microbial products containing salts of phosphorous acid are marketed in Australia as 'Yates Anti-Rot'; and in the United States of America, for example, aluminum salts of the monoethyl ester of phosphorous acid (known generically as 'Fosetyl-Al') are sold under the trade name 'Aliette'. Phosphorous acid and its salts, unlike phosphoric acid, are somewhat toxic and should be handled carefully.[10][11]
Organic derivatives
The IUPAC (mostly organic) name is phosphonic acid. This nomenclature is commonly reserved for substituted derivatives, that is, organic group bonded to phosphorus, not simply an ester. For example, (CH3)PO(OH)2 is "methylphosphonic acid", which may of course form "methylphosphonate" esters.
References
- ^ http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=215112&brand=SIAL&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsial%2F215112%3Flang%3Den
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. Electronic version..
- ^ Xi, Chanjuan; Liu, Yuzhou; Lai, Chunbo; Zhou, Lishan (2004). "Synthesis of molybdenum complex with novel P(OH)3 ligand based on the one-pot reaction of Mo(CO)6 with HP(O)(OEt)2 and water". Inorganic Chemistry Communications. 7 (11): 1202. doi:10.1016/j.inoche.2004.09.012.
- ^ Sokolov, M. N.; Chubarova, E. V.; Kovalenko, K. A.; Mironov, I. V.; Virovets,, A. V.; Peresypkina,, E. V.; Fedin, V. P. (2005). "Stabilization of tautomeric forms P(OH)3 and HP(OH)2 and their derivatives by coordination to palladium and nickel atoms in heterometallic clusters with the Mo
3MQ4+
4 core (M = Ni, Pd; Q = S, Se)". Russian Chemical Bulletin. 54 (3): 615. doi:10.1007/s11172-005-0296-1.{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Larson, John W.; Pippin, Margaret (1989). "Thermodynamics of ionization of hypophosphorous and phosphorous acids. Substituent effects on second row oxy acids". Polyhedron. 8: 527–530. doi:10.1016/S0277-5387(00)80751-2.
- ^ CRC Handbook of Chemistry and Physics (87th ed.). p. 8–42.
- ^ Novosad, Josef (1994). Encyclopedia of Inorganic Chemistry. John Wiley and Sons. ISBN 0-471-93620-0.
- ^ Gokhale, S. D.; Jolly, W. L. (1967). "Phosphine". Inorganic Syntheses. 9: 56–58. doi:10.1002/9780470132401.ch17.
- ^ Organic Labs. Product label for 'Exel LG,' Retrieved April 9, 2007.
- ^ Yates, a Division of Orica Australia Pty Ltd. “MSDS ('Yates Anti Rot Phosacid Systemic Fungicide').” Version 1. SH&E Shared Services, Orica. Homebush, NSW (Australia): April 4, 2005 (retrieved from www.orica.com April 9, 2007).
- ^ US EPA. “Fosetyl-Al (Aliette): Reregistration Eligibility Decision (RED) Fact Sheet.” Office of Pesticide Programs, US EPA. Washington, DC (USA): 1994 (retrieved from www.epa.gov April 9, 2007).
Further reading
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- Corbridge., D. E. C. Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier. ISBN 0-444-89307-5.
- Lee, J.D. Concise Inorganic Chemistry. Oxford University Press. ISBN 978-81-265-1554-7.