Phosphorus heptabromide
Appearance
| |

| |
| Names | |
|---|---|
| Other names
Tetrabromophosphonium tribromide
Perbromophosphonium tribromide Phosphorus heptabromide | |
| Identifiers | |
3D model (JSmol)
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| Br7P | |
| Molar mass | 590.302 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
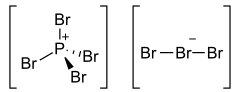
Phosphorus heptabromide is an inorganic compound with the formula PBr7. It is one of the phosphorus bromides. At normal conditions, it forms red prismatic crystals. PBr7 can be prepared by the sublimation of a mixture of phosphorus pentabromide and bromine.[1]
The structure consists of a PBr4+ cation paired with a tribromide (Br3–) anion, and the tribromide is non-symmetric.[2]
See also
References
- ^ T. E. (Thomas Edward) Thorpe. A dictionary of applied chemistry (Volume 4)
- ^ Breneman, G. L.; Willett, R. D. (1967). "The crystal structure of phosphorus heptabromide, PBr7". Acta Crystallographica. 23 (3): 467–471. doi:10.1107/S0365110X67002981.
