Propionate
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Propionate
| |
| Other names
Propanoate, Propanoic acid, ion(1-)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H5O2 | |
| Molar mass | 73.071 g·mol−1 |
| Appearance | Colorless, oily liquid[dubious – discuss] |
| Density | 0.993 g/mL at 20°C[1][dubious – discuss] |
| Melting point | −21.5 °C (−6.7 °F; 251.7 K)[1][dubious – discuss] |
| Boiling point | 141.1 °C (286.0 °F; 414.2 K)[1][dubious – discuss] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable, Corrosive |
| Flash point | 52 °C (126 °F; 325 K)[1][dubious – discuss] |
| 465 °C (869 °F; 738 K)[1][dubious – discuss] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
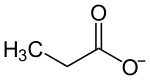
The propionate or propanoate ion is C2H5COO− (the conjugate base of propionic acid).
A propionic or propanoic compound is a small salt or ester of propionic acid. In these compounds, propionate is often written in shorthand, as CH3CH2CO2 or simply EtCO2.
Propionates should not be confused with propenoates (commonly known as acrylates), the ions/salts/esters of propenoic acid (also known as 2-propenoic acid or acrylic acid).
Examples
- Sodium propionate, NaC2H5CO2
- Methyl propionate, (C2H5(CO)OCH3)
- Calcium propionate, Ca(C2H5CO2)2
- Potassium propionate, KC2H5CO2
- Fluticasone propionate, C25H31F3O5S
References
