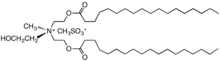
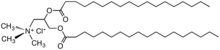
Quaternary ammonium cation
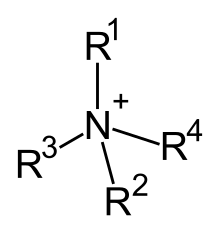
Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR4+, R being an alkyl group or an aryl group.[1] Unlike the ammonium ion (NH4+) and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations.
Synthesis
Quaternary ammonium compounds are prepared by the alkylation of tertiary amines with a halocarbon. In older literature this is often called a Menshutkin reaction, however modern chemists usually refer to it simply as quaternization.[2] The reaction can be used to produce a compound with unequal alkyl chain lengths; for example when making cationic surfactants one of the alkyl groups on the amine is typically longer than the others.[3] A typical synthesis is for benzalkonium chloride from a long-chain alkyldimethylamine and benzyl chloride:
- CH3(CH2)nN(CH3)2 + ClCH2C6H5 → [CH3(CH2)nN(CH3)2CH2C6H5]+Cl−
Reactions
Quaternary ammonium cations are unreactive toward even strong electrophiles, oxidants, and acids. They also are stable toward most nucleophiles. The latter is indicated by the stability of the hydroxide salts such as tetramethylammonium hydroxide and tetrabutylammonium hydroxide. Because of their resilience, many unusual anions have been isolated as the quaternary ammonium salts. Examples include tetramethylammonium pentafluoroxenate, containing the highly reactive pentafluoroxenate (XeF5−) ion. Permanganate can be solubilized in organic solvents, when deployed as its NBu4+ salt.[4][5]
With exceptionally strong bases, quat cations degrade. They undergo Sommelet–Hauser rearrangement[6] and Stevens rearrangement,[7] as well as dealkylation under harsh conditions. Quaternary ammonium cations containing N-C-C-H units can also undergo the Hofmann Elimination and Emde degradation.
Applications
Quaternary ammonium salts are used as disinfectants, surfactants, fabric softeners, and as antistatic agents (e.g. in shampoos). In liquid fabric softeners, the chloride salts are often used. In dryer anticling strips, the sulfate salts are often used. Spermicidal jellies also contain quaternary ammonium salts.
As antimicrobials
Quaternary ammonium compounds have also been shown to have antimicrobial activity.[8] Certain quaternary ammonium compounds, especially those containing long alkyl chains, are used as antimicrobials and disinfectants. Examples are benzalkonium chloride, benzethonium chloride, methylbenzethonium chloride, cetalkonium chloride, cetylpyridinium chloride, cetrimonium, cetrimide, dofanium chloride, tetraethylammonium bromide, didecyldimethylammonium chloride and domiphen bromide. Also good against fungi, amoebas, and enveloped viruses,[9] quaternary ammonium compounds are believed to act by disrupting the cell membrane.[citation needed] Quaternary ammonium compounds are lethal to a wide variety of organisms except endospores, Mycobacterium tuberculosis and non-enveloped viruses.
Quaternary ammonium compounds are cationic detergents, as well as disinfectants, and as such can be used to remove organic material. They are very effective in combination with phenols. Quaternary ammonium compounds are deactivated by anionic detergents (including common soaps). Also, they work best in soft waters[citation needed]. Effective levels are at 200 ppm. They are effective at temperatures up to 212 °F (100 °C).
Quaternary ammonium salts are commonly used in the foodservice industry as sanitizing agents.
As phase transfer catalysts
In organic chemistry, quaternary ammonium salts are employed as phase transfer catalysts (PTCs). Such catalysts accelerate reactions between reagents dissolved in immiscible solvents. The highly reactive reagent dichlorocarbene is generated via PTC by reaction of chloroform and aqueous sodium hydroxide.
Fabric softeners
In the 1950s, distearyldimethylammonium chloride (DHTDMAC), was introduced as a fabric softener. This compound was discontinued because the cation biodegrades too slowly. Contemporary fabric softeners are based on salts of quaternary ammonium cations where the fatty acid is linked to the quaternary center via ester linkages; these are commonly referred to as betaine-esters or ester-quats and are susceptible to degradation, e.g., by hydrolysis.[10] Characteristically, the cations contain one or two long alkyl chains derived from fatty acids linked to an ethoxylated ammonium salt.[11] Other cationic compounds can be derived from imidazolium, guanidinium, substituted amine salts, or quaternary alkoxy ammonium salts.[12]
- Cationic surfactants used as fabric softeners
-
Distearyldimethylammonium chloride, an early generation fabric softener with low biodegradability that was phased out.
-
Another diesterquat, a contemporary fabric softener.
-
Diethylester dimethyl ammonium chloride used as a fabric softener.
-
Another diesterquat used as a fabric softener.
Osmolytes
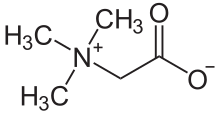
Quaternary ammonium compounds are present in osmolytes, specifically glycine betaine, which stabilize osmotic pressure in cells.[13]
Plant growth retardants
Cycocel (chlormequat chloride) reduces plant height by inhibiting the production of gibberellins, the primary plant hormones responsible for cell elongation. Therefore, their effects are primarily on stem, petiole and flower stalk tissues. Lesser effects are seen in reductions of leaf expansion, resulting in thicker leaves with darker green color.[14]
Health effects
Quaternary ammonium compounds can display a range of health effects, amongst which are mild skin and respiratory irritation [15] up to severe caustic burns on skin and gastro-intestinal lining (depending on concentration), gastro-intestinal symptoms (e.g., nausea and vomiting), coma, convulsions, hypotension and death.[16]
They are thought to be the chemical group responsible for anaphylactic reactions that occur with use of neuromuscular blocking drugs during general anaesthesia in surgery.[17] Quaternium-15 is the single most often found cause of allergic contact dermatitis of the hands (16.5% in 959 cases)[18]
Possible reproductive effects in laboratory animals
Quaternary ammonium-based disinfectants (Virex and Quatricide) were tentatively identified as the most probable cause of jumps in birth defects and fertility problems in caged lab mice.[19] See also Hunt and Hrubek (Reproductive Toxicology, 50:163-70, 2014).
Quantification
The quantification of quaternary ammonium compounds in environmental and biological samples is problematic using conventional chromatography techniques because the compounds are highly soluble in water. While analyzing them by liquid chromatography coupled tandem mass spectrometry it has been found that they follow an exception rule. Under standard electrospray ionization (ESI) conditions, mono- and di-quaternary ammonium compounds form molecular ions with the formula of rather than .[clarification needed] Formation of is observed for di-quaternary ammonium compounds (like diquat) as precursor ion and as product ion due to the loss of one of the quaternary charge during CID. In di-quaternary ammonium compounds, this process can also result in the formation of fragment ions with higher mass as compared to their precursor ion. Hydrophilic interaction liquid chromatographic separation has been reported to demonstrate a successful separation of quaternary ammonium compounds for their quantification in ESI-MS/MS with higher precision.[20]
See also
- Benzalkonium chloride, benzethonium chloride, methylbenzethonium chloride, cetalkonium chloride, cetylpyridinium chloride, cetrimonium, cetrimide, dofanium chloride, tetraethylammonium bromide, and domiphen bromide – antimicrobial ingredients found in various over-the-counter products
- Diquat -diquaternary ammonium compound recognized as a contact herbicide
- Carnitine
- Cetyl trimethylammonium bromide (CTAB), stearalkonium chloride – cationic surfactants commonly used in toiletries
- Choline
- Cocamidopropyl betaine (CAPB), a common Zwitterionic surfactant used ubiquitously in toiletries
- Denatonium, the most bitter compound known
- Dimethyldioctadecylammonium chloride
- Paraquat, an herbicide
- Polyquaternium, designations for quaternary ammonium-containing polymers used for personal care products
- Quaternary ammonium muscle relaxants
- Silicone quaternary amine
- Tetra-n-butylammonium bromide and Aliquat 336, common phase transfer catalysts
- Tetramethylammonium chloride
- Tetramethylammonium hydroxide
- Tetramethylammonium pentafluoroxenate, containing the unusual pentagonal pentafluoroxenate (XeF5−) ion
- Triazene cleavage
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "quaternary ammonium compounds". doi:10.1351/goldbook.Q05003
- ^ Smith, Michael B.; March, Jerry (2001), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (5th ed.), New York: Wiley-Interscience, ISBN 0-471-58589-0
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Kosswig, K. "Surfactants" in Ullmann’s Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_747.
- ^ Arthur W. Herriott (1977). "Purple benzene: Solubilization of anions in organic solvents". J. Chem. Educ. 54 (4): 229. doi:10.1021/ed054p229.1.
- ^ Doheny, Anthony J., Jr. and Ganem, Bruce (1980). "Purple benzene revisited". J. Chem. Educ. 57 (4): 308. doi:10.1021/ed057p308.1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ W. R. Brasen; C. R. Hauser (1963). "2-Methylbenzyldimethylamine". Organic Syntheses; Collected Volumes, vol. 4, p. 585.
- ^ Pine, Stanley H. (2011). "Organic Reactions". doi:10.1002/0471264180.or018.04. ISBN 0471264180.
{{cite journal}}:|chapter=ignored (help); Cite journal requires|journal=(help) - ^ Jia, Zhishen; Shen, Dongfeng; Xu, Weiliang (2001). "Synthesis and antibacterial activities of quaternary ammonium salt of chitosan". Carbohydrate Research. 333 (1): 1–6. doi:10.1016/S0008-6215(01)00112-4. PMID 11423105.
- ^ Specific Antimicrobials, outline of lecture by Stephen T. Abedon, Ohio State U., URL accessed Dec 2008.
- ^ Hellberg, Per-Erik; Bergström, Karin; Holmberg, Krister (January 2000). "Cleavable surfactants". Journal of Surfactants and Detergents. 3 (1): 81–91. doi:10.1007/s11743-000-0118-z.
- ^ "Henkel Consumer Info". Henkelconsumerinfo.com. Retrieved 2009-06-04.
- ^ E. Smulders, E. Sung "Laundry Detergents, 2. Ingredients and Products" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.o15_013
- ^ Sleator, Roy D.; Wouters, Jeroen; Gahan, Cormac G. M.; Abee, Tjakko; Hill, Colin (2001). "Analysis of the Role of OpuC, an Osmolyte Transport System, in Salt Tolerance and Virulence Potential of Listeria monocytogenes". Appl. Environ. Microbiol. 67: 2692–2698. doi:10.1128/AEM.67.6.2692-2698.2001.
- ^ http://users.in.gr/dimpet/thesis/growth_retardants.htm Wageningen Agricultural University, The Netherlands
- ^ Bello, Anila; Quinn, Margaret M; Perry, Melissa J; Milton, Donald K (2009). "Characterization of occupational exposures to cleaning products used for common cleaning tasks-a pilot study of hospital cleaners". Environmental Health. 8: 11. doi:10.1186/1476-069X-8-11. PMC 2678109. PMID 19327131.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Quaternary ammonium (PIM G022)
- ^ Harper, NJ; Dixon, T; Dugué, P; Edgar, DM; Fay, A; Gooi, HC; Herriot, R; Hopkins, P; Hunter, JM; Mirakian, R; Pumphrey, RS; Seneviratne, SL; Walls, AF; Williams, P; Wildsmith, JA; Wood, P; Nasser, AS; Powell, RK; Mirakhur, R; Soar, J; Working Party of the Association of Anaesthetists of Great Britain Ireland (2009). "Suspected anaphylactic reactions associated with anaesthesia". Anaesthesia. 64 (2): 199–211. doi:10.1111/j.1365-2044.2008.05733.x. PMC 3082210. PMID 19143700.
- ^ Warshaw, EM; Ahmed, RL; Belsito, DV; Deleo, VA; Fowler Jr, JF; Maibach, HI; Marks Jr, JG; Toby Mathias, CG; Pratt, MD; Rietschel, RL; Sasseville, D; Storrs, FJ; Taylor, JS; Zug, KA; North American Contact Dermatitis Group (2007). "Contact dermatitis of the hands: Cross-sectional analyses of North American Contact Dermatitis Group Data, 1994-2004". Journal of the American Academy of Dermatology. 57 (2): 301–14. doi:10.1016/j.jaad.2007.04.016. PMID 17553593.
- ^ Hunt, P (June 2008). "Lab disinfectant harms mouse fertility. Patricia Hunt interviewed by Brendan Maher". Nature. 453 (7198): 964. doi:10.1038/453964a. PMID 18563110.
- ^ Thirumurthy Velpandiana; Jayabalan Nirmala; Beauty Aroraa; Alok Kumar Ravia; Ankita Kotnalaa (October 2012). "Understanding the Charge Issues in Mono and di-Quaternary Ammonium Compounds for Their Determination by LC/ESI-MS/MS". Analytical Letters. 45 (16): 2367–2376. doi:10.1080/00032719.2012.693140.
External links
- Toxicities of quaternary ammonium
- Chang Zhang, Fang Cui, Guang-ming Zeng, Min Jiang, Zhong-zhu Yang, Zhi-gang Yu, Meng-ying Zhu, Liu-qing Shen: Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Science of The Total Environment, Volumes 518–519, 15 June 2015, Pages 352–362, doi:10.1016/j.scitotenv.2015.03.007