SCIMP protein
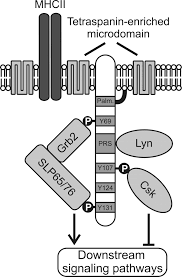
SLP65/SLP76, Csk-interacting membrane protein, termed SCIMP, belongs to family of transmembrane adaptor proteins (TRAP) which do not directly associate with a receptor, such as LAT, NTAL, LIME or LAX.[1][2][3][4] SCIMP is expressed in antigen-presenting cells (APC), namely B cells, bone marrow-derived dendritic cells and macrophages.
Structure and interactions
[edit]Like other TRAPs, SCIMP has negligible extracellular domain and transmembrane domain followed by intracellular domain, containing several tyrosines and one proline-rich region (PRR). Upon phosphorylation, these tyrosines serve as docking domains for SH2 domains containing proteins. In a contrast to phospho-tyrosines, proline rich regions are generally less susceptible to post-translation modifications and they are rather targets of constitutive interactions with SH3 domains containing proteins.[5] It has been shown that SCIMP interact via SH2 domains with Csk kinase, negative regulator of Src family kinases, but also with Slp65/76 and Grb2 adaptors, which are key pro-signalling soluble adaptor proteins in lymphocyte signalling network. SCIMP is constitutively associated with Lyn kinase via SH3 domain.
Membrane localization
[edit]Some of TRAPs are palmitoylated in a border region between transmembrane and intracellular domain. The aliphatic chain of Palmitic acid is anchored to the membrane bilayer and thus influence protein targeting to membrane microdomains. SCIMP is also palmitoylated and is associated with tetraspanin-enriched mircrodomains (TEMs). TEMs, unlike lipid rafts, are based more on protein-protein interactions than lipid-lipid/lipid-protein interactions.[6] One of the resident proteins in TEMs is MHC class II molecule. SCIMP is present in the immunological synapse during antigen presentation between a T cell and an antigen-presenting cell (APC).
In vitro studies and putative function
[edit]SCIMP becomes strongly phosphorylated after MHC II stimulation. Studies performed with fusion protein CD25-SCIMP showed its ability to induce calcium release and Erk phosphorylation upon anti-CD25 antibody treatment. The calcium release was even stronger in CD25-SCIMP mutant protein in binding side for Csk. Indicating negative feedback loop performed by Csk kinase. Fusion proteins are commonly used in order to study signalling ability of proteins with a small extracellular domain hidden for antibody in membrane glycocalix. However knock down of SCIMP didn´t influence calcium release after anti MHC II antibody treatment, but only decrease level of Erk phosphorylation in longer time point (10 min.)[7]
References
[edit]- ^ Brdicka, T.; Imrich, M.; Angelisova, P.; Brdickova, N.; Horvath, O.; Spicka, J.; Hilgert, I.; Luskova, P.; Draber, P.; Novak, P.; Engels, N.; Wienands, J.; Simeoni, L.; Osterreicher, J.; Aguado, E.; Malissen, M.; Schraven, B.; Horejsi, V. (9 December 2002). "Non-T Cell Activation Linker (NTAL): A Transmembrane Adaptor Protein Involved in Immunoreceptor Signaling". Journal of Experimental Medicine. 196 (12): 1617–1626. doi:10.1084/jem.20021405. PMC 2196071. PMID 12486104.
- ^ Hur, E. M.; Son, M.; Lee, O.-H.; Choi, Y. B.; Park, C.; Lee, H.; Yun, Y. (10 November 2003). "LIME, a Novel Transmembrane Adaptor Protein, Associates with p56lck and Mediates T Cell Activation". Journal of Experimental Medicine. 198 (10): 1463–1473. doi:10.1084/jem.20030232. PMC 2194117. PMID 14610044.
- ^ Weber, JR (6 April 1998). "Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells". J Exp Med. 187 (7): 1157–1161. doi:10.1084/jem.187.7.1157. PMC 2212210. PMID 9529333.
- ^ Zhu, M. (30 September 2002). "Molecular Cloning of a Novel Gene Encoding a Membrane-associated Adaptor Protein (LAX) in Lymphocyte Signaling". Journal of Biological Chemistry. 277 (48): 46151–46158. doi:10.1074/jbc.M208946200. PMID 12359715.
- ^ Williamson, MP (15 January 1994). "The structure and function of proline-rich regions in proteins". Biochem. J. 297 ( Pt 2) (2): 249–60. doi:10.1042/bj2970249. PMC 1137821. PMID 8297327.
- ^ Stepanek, Ondrej; Draber, Peter; Horejsi, Vaclav (May 2014). "Palmitoylated transmembrane adaptor proteins in leukocyte signaling". Cellular Signalling. 26 (5): 895–902. doi:10.1016/j.cellsig.2014.01.007. PMID 24440308.
- ^ Draber, P.; Vonkova, I.; Stepanek, O.; Hrdinka, M.; Kucova, M.; Skopcova, T.; Otahal, P.; Angelisova, P.; Horejsi, V.; Yeung, M.; Weiss, A.; Brdicka, T. (19 September 2011). "SCIMP, a Transmembrane Adaptor Protein Involved in Major Histocompatibility Complex Class II Signaling". Molecular and Cellular Biology. 31 (22): 4550–4562. doi:10.1128/MCB.05817-11. PMC 3209250. PMID 21930792.
