Saline (medicine)
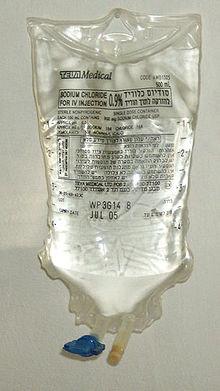 Saline solution for intravenous infusion. The white port at the base of the bag is where additives can be injected. The port with the blue cover is where the bag is spiked with an infusion set. | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | FDA Professional Drug Information |
| Routes of administration | intravenous, topical, subcutaneous |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
Saline, also known as saline solution, is a mixture of sodium chloride in water and has a number of uses in medicine.[1] Applied to the affected area it is used to clean wounds, help remove contact lenses, and help with dry eyes.[2] By injection into a vein it is used to treat dehydration such as from gastroenteritis and diabetic ketoacidosis.[2] It is also used to dilute other medications to be given by injection.[1]
Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium.[1][2] In those with long standing low blood sodium excessive use may result in osmotic demyelination syndrome.[2] Saline is in the crystalloid family of medications.[3] It is most commonly used as a sterile 9 g of salt per litre (0.9%) solution, known as normal saline.[1] Higher and lower concentrations may also occasionally be used.[4][5] Saline has a pH of 5.5 making it acidic.[6]
The medical use of saline began around 1831.[7] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[8] The wholesale cost in the developing world is about 0.60 to 4.20 USD per liter of normal saline.[9]
Concentrations

Concentrations vary from low to normal to high. High concentrations are used rarely in medicine but frequently in molecular biology.
Normal
Normal saline (NSS, NS or N/S) is the commonly used phrase for a solution of 0.90% w/v of NaCl, 308 mOsm/L or 9.0 g per litre. Less commonly, this solution is referred to as physiological saline or isotonic saline (because it closely approximates isotonic, that is, physiologically normal, solution); although neither of those names is technically accurate (because normal saline is not exactly like blood serum), they convey the practical effect usually seen: good fluid balance with minimal hypotonicity or hypertonicity. NS is used frequently in intravenous drips (IVs) for patients who cannot take fluids orally and have developed or are in danger of developing dehydration or hypovolemia. NS is also used for aseptic purpose. NS is typically the first fluid used when hypovolemia is severe enough to threaten the adequacy of blood circulation, and has long been believed to be the safest fluid to give quickly in large volumes. However, it is now known that rapid infusion of NS can cause metabolic acidosis.[10]
The solution is 9 grams of sodium chloride (NaCl) dissolved in water, to a total volume of 1000 ml (weight per unit volume(w/v)). The mass of 1 millilitre of normal saline is 1.0046 gram at 22 °C.[11][12] The molecular weight of sodium chloride is approximately 58.5 grams per mole, so 58.5 grams of sodium chloride equals 1 mole. Since normal saline contains 9 grams of NaCl, the concentration is 9 grams per litre divided by 58.5 grams per mole, or 0.154 mole per litre. Since NaCl dissociates into two ions – sodium and chloride – 1 molar NaCl is 2 osmolar. Thus, NS contains 154 mEq/L of Na+ and Cl−. It has a slightly higher degree of osmolarity (i.e. more solute per litre) than blood (However, if you take into account the osmotic coefficient, a correction for non-ideal solutions, then the saline solution is much closer to isotonic. Osmotic coefficient of NaCl is about 0.93; therefore 0.154 × 1000 × 2 × .93 = 286.44) Nonetheless, the osmolarity of normal saline is a close approximation to the osmolarity of NaCl in blood.
One litre of 0.9% Saline contains:
Usage
For medical purposes, saline is often used to flush wounds and skin abrasions. Normal saline will not burn or sting when applied.
Saline is also used in I.V. therapy, intravenously supplying extra water to rehydrate patients or supplying the daily water and salt needs ("maintenance" needs) of a patient who is unable to take them by mouth. Because infusing a solution of low osmolality can cause problems such as hemolysis, intravenous solutions with reduced saline concentrations typically have dextrose (glucose) added to maintain a safe osmolality while providing less sodium chloride.
The amount of normal saline infused depends largely on the needs of the patient (e.g. ongoing diarrhea or heart failure).
Saline is also often used for nasal washes to relieve some of the symptoms of the common cold.[13] The solution exerts a softening and loosening influence on the mucus to make it easier to wash out and clear the nasal passages for both babies[14] and adults.[15][16] In this case "home-made" saline may be used: this is made by dissolving approximately half a teaspoon of table salt into 240ml (approx. 8 ounces) of clean tap water.[17] In very rare instances, amoeba Naegleria fowleri infection can occur if amoeba enters the body through the nose, therefore water used for nasal irrigation should be sterile.[18] Note that clean tap water is not necessarily a sterile liquid.
Eyes
Eye drops are saline-containing drops used on the eye. Depending on the condition being treated, they may contain steroids, antihistamines, sympathomimetics, beta receptor blockers, parasympathomimetics, parasympatholytics, prostaglandins, non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics or topical anesthetics. Eye drops sometimes do not have medications in them and are only lubricating and tear-replacing solutions.
Syringe designed saline drops (e.g. Wallace Cameron Ultra Saline Minipod) are distributed in modern needle-exchange programmes as drugs efficiently can be administered either by injection, or ophthalmic, which is compared to intravenous use; By demonstration, the elimination of latanoprost acid from plasma is rapid (half-life 17 minutes) after either ophthalmic or intravenous administration.[19] However, ophthalmic use is done with sterile filtered drugs that is potent in quite small doses, and with adjusted acidity of pH 7.0–7.5 after the drug has been added, to avoid eye damage. The human eye has a pH of approximately 7.5, water has 7.0.[20]
Hypertonic saline
Hypertonic saline—7% NaCl solutions are considered mucoactive agents and thus are used to hydrate thick secretions (mucus) in order to make it easier to cough up and out (expectorate). 3% hypertonic saline solutions are also used in critical care settings, acutely increased intracranial pressure, or severe hyponatremia.[21] Inhalation of hypertonic saline has also been shown to help in other respiratory problems, specifically bronchiolitis.[22] Hypertonic saline is currently recommended by the Cystic Fibrosis Foundation as a primary part of a cystic fibrosis treatment regimen.[23]
An 11% solution of xylitol with 0.65% saline stimulates the washing of the nasopharynx and has an effect on the nasal pathogenic bacteria. This has been used in complementary and alternative medicine.[24]
Other
Other concentrations commonly used include:
- Half-normal saline (0.45% NaCl), often with "D5" (5% dextrose), contains 77 mEq/L of Na and Cl and 50 g/L dextrose.
- Quarter-normal saline (0.22% NaCl) has 39 mEq/L of Na and Cl and almost always contains 5% dextrose for osmolality reasons. It can be used alone in Neonatal Intensive Care Units.
- Hypertonic saline may be used in perioperative fluid management protocols to reduce excessive intravenous fluid infusions and lessen pulmonary complications.[25] Hypertonic saline is used in treating hyponatremia and cerebral edema Rapid correction of hyponatremia via hypertonic saline, or via any saline infusion > 40 mmol/L (Na+ having a valence of 1, 40 mmol/L = 40 mEq/L) greatly increases risk of central pontine myelinolysis (CPM), and so requires constant monitoring of patient response. Water privation combined with diuretic block does not produce as much risk of CPM as saline administration does; however, it does not correct hyponatremia as rapidly as administration of hypertonic saline does. Due to hypertonicity, administration may result in phlebitis and tissue necrosis. As such, concentrations greater than 3% NaCl should normally be administered via a central venous catheter, also known as a 'central line'. Such hypertonic saline is normally available in two strengths, the former of which is more commonly administered:
- 3% NaCl has 513 mEq/L of Na and Cl.
- 5% NaCl has 856 mEq/L of Na and Cl.
- NaCl solutions that are less commonly used are 7% (1200 mEq/L) and 23.4% (approx 4000 mEq/L), both of which are used (also via central line), often in conjunction with supplementary diuretics, in the treatment of traumatic brain injury.[26]
- Dextrose (glucose) 4% in 0.18% saline is used sometimes for maintenance replacement.
Solutions with added ingredients
In medicine, common types of salines include:
- Lactated Ringer's solution
- Acetated Ringer's solution
- Intravenous sugar solutions
- 5% dextrose in normal saline (D5NS)
- 10% dextrose in normal saline (D10NS)
- 5% dextrose in half-normal saline (D5HNS)
- 10% dextrose in half-normal saline (D10HNS)
And in cell biology, in addition to the above the following are used:
- Phosphate buffered saline (PBS) (recipes from Dulbecco = D-PBS, Galfre, Kuchler, Ausubel etc.)
- TRIS-buffered saline (TBS) (recipes from Goldsmith, Ausubel etc.)
- Hank's balanced salt solution (HBSS)
- Earle's balanced salt solution (EBSS)
- Standard saline citrate (SSC)
- HEPES-buffered saline (HBS) (recipes from Dittmar, Liu, Ausubel etc.)
- Gey's balanced salt solution (GBSS)
History
Saline was believed to have originated during the Indian Blue cholera pandemic that swept across Europe in 1831. William Brooke O'Shaughnessy, a recent graduate of Edinburgh Medical School, proposed in an article to medical journal The Lancet to inject cholera patients with highly oxygenated salts to treat the "universal stagnation of the venous system and rapid cessation of arterialisation of the blood" seen in severely dehydrated cholera patients.[27] He found his treatment harmless in dogs, and his proposal was soon adopted by the physician Thomas Latta in treating cholera patients to beneficial effect. In the following decades, variations and alternatives to Latta's solution were tested and used in treating cholera patients. These solutions contained a range of concentrations of sodium, chloride, potassium, carbonate, phosphate, and hydroxide. The breakthrough in achieving physiological concentrations was accomplished by Sydney Ringer in the early 1880s,[28] when he determined the optimal salt concentrations to maintain the contractility of frog heart muscle tissue. Normal saline is considered a descendant of the pre-Ringer solutions, as Ringer's findings were not adopted and widely used until decades later. The term "normal saline" itself appears to have little historical basis, except for studies done in 1882–83 by Dutch physiologist Hartog Jacob Hamburger; these in vitro studies of red cell lysis suggested incorrectly that 0.9% was the concentration of salt in human blood (rather than 0.6%, the true concentration).[29]
Normal saline has become widely used in modern medicine, but due to the mismatch with real blood, other solutions have proved better. The 2018 publication of a randomized, controlled trial with 15,000 patients showed that lactated Ringer's solution reduced mortality risk in intensive care unit patients by 1% compared to normal saline, which given the large number of patients is a significant reduction.[30]
Society and culture
Coconut water has been used in place of normal saline in areas without access to normal saline.[31] Its use, however, has not been well studied.[31]
See also
References
- ^ a b c d "Sodium Chloride Injection - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 14 January 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 683, 770. ISBN 9780857111562.
- ^ Marini, John J.; Wheeler, Arthur P. (2010). Critical Care Medicine: The Essentials. Lippincott Williams & Wilkins. p. 54. ISBN 9780781798396. Archived from the original on 2017-09-18.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Hypertonic Saline - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 18 January 2017. Retrieved 14 January 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Pestana, Carlos (2000). Fluids and Electrolytes in the Surgical Patient. Lippincott Williams & Wilkins. p. 11. ISBN 9780781724258. Archived from the original on 2017-09-18.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Reddi, BA (2013). "Why is saline so acidic (and does it really matter?)". International journal of medical sciences. 10 (6): 747–50. doi:10.7150/ijms.5868. PMID 23630439.
- ^ Bozzetti, Federico; Staun, Michael; Gossum, Andre van (2014). Home Parenteral Nutrition, 2nd Edition. CABI. p. 4. ISBN 9781780643113. Archived from the original on 2017-09-18.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived from the original (PDF) on 13 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Sodium Chloride in Water". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ^ Prough, DS; Bidani, A (1999). "Hyperchloremic metabolic acidosis is a predictable consequence of intraoperative infusion of 0.9% saline". Anesthesiology. 90 (5): 1247–1249. doi:10.1097/00000542-199905000-00003. PMID 10319767.
- ^ Fluid Density Calculator Archived 2009-09-16 at the Wayback Machine. Earthwardconsulting.com. Retrieved on 2011-02-27.
- ^ Water Density Calculator Archived 2010-01-22 at the Wayback Machine. Csgnetwork.com. Retrieved on 2011-02-27.
- ^ Cure a cold: Saline Nasal drops Archived 2013-01-16 at the Wayback Machine
- ^ Blocked Nose in Babies ('Snuffles') at Patient UK
- ^ What does saline nasal spray do?
- ^ Tixylix saline nasal drops: How does it work Archived 2012-11-01 at the Wayback Machine
- ^ Homemade Saline Nose Drops Archived 2012-11-27 at the Wayback Machine at Food.com
- ^ "CDC - Naegleria - Frequently Asked Questions (FAQs)". Archived from the original on 2012-03-20. Retrieved April 2012.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Latanoprost Drug Information, Professional". Drugs.com. Archived from the original on 2013-03-29. Retrieved 2012-05-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "pH Value Eye Drops". Clear-lenses.com. 2004-08-22. Archived from the original on 2012-05-16. Retrieved 2012-05-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Reeves EP et al (2011). "Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis." Am J Respir Crit Care Med. 2011 Jun 1;183(11):1517-23. doi: 10.1164/rccm.201101-0072OC. PMID 21330456 Archived 2016-03-29 at the Wayback Machine
- ^ Principi T, Komar L (2011). "A critical review of "a randomized trial of nebulized 3% hypertonic saline with epinephrine in the treatment of acute bronchiolitis in the emergency department."". J Popul Ther Clin Pharmacol. 18 (2): e273–4. PMID 21633141.
- ^ O'Connell OJ, O'Farrell C, Harrison MJ, Eustace JA, Henry MT, Plant BJ (2011). "Nebulized hypertonic saline via positive expiratory pressure versus via jet nebulizer in patients with severe cystic fibrosis". Respir Care. 56 (6): 771–5. doi:10.4187/respcare.00866. PMID 21333079.
- ^ Jones, Alonzo. "Intranasal Xylitol, Recurrent Otitis Media, and Asthma: Report of Three Cases*". Nasal xylitol, from Clinical Practice of Alternative Medicine. Alonzo H. Jones, DO. Archived from the original on 8 May 2014. Retrieved 7 May 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Vivian McAlister, Karen E. A. Burns, Tammy Znajda, and Brian Church. "Hypertonic Saline for Peri-operative Fluid Management" Cochrane Database of Systematic Reviews.1 (2010): CD005576 Available at: "Archived copy". Archived from the original on 2011-07-06. Retrieved 2011-02-06.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ Access Archived 2010-11-19 at the Wayback Machine. Medscape. Retrieved on 2011-02-27.
- ^ O'Shaugnessy, WB (1831). "Proposal for a new method of treating the blue epidemic cholera by the injection of highly-oxygenated salts into the venous system". Lancet. 17 (432): 366–71. doi:10.1016/S0140-6736(02)94163-2.
- ^ Kenneth M Sutin; Marino, Paul L. (2007). "The ICU book" Archived 2017-09-18 at the Wayback Machine. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-4802-X.
- ^ Awad, Sherif; Allison Simon P; Lobo Dileep N (2008). "The history of 0.9% saline". Clinical Nutrition. 27 (2): 179–88. doi:10.1016/j.clnu.2008.01.008. PMID 18313809.
- ^ Why Did Sterile Salt Water Become The IV Fluid Of Choice?
- ^ a b Campbell-Falck, D; Thomas, T; Falck, TM; Tutuo, N; Clem, K (January 2000). "The intravenous use of coconut water". The American journal of emergency medicine. 18 (1): 108–11. doi:10.1016/s0735-6757(00)90062-7. PMID 10674546.
