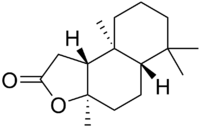
Sclareolide
Appearance
| |
| Names | |
|---|---|
| IUPAC name
3a,6,6,9a-tetramethyl-1,4,5,5a,7,8,9,9b-octahydronaphtho[8,7-d]furan-2-one
| |
| Other names
Norambreinolide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.427 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H26O2 | |
| Molar mass | 250.382 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sclareolide is a sesquiterpene lactone natural product derived from various plant sources including Salvia sclarea, Salvia yosgadensis,[1] and cigar tobacco.[2] It is a close analog of sclareol, a plant antifungal compound.[3]
It is used as a fragrance in cosmetics and has been more recently marketed as a weight loss supplement, though there is no clinical evidence to support this effect.
References
- ^ Topcu, Guelacti; Ulubelen, Ayhan; Tam, Timothy Chit-Ming; Che, Chun-Tao (1996). "Norditerpenes and Norsesterterpenes from Salvia yosgadensis". Journal of Natural Products. 59 (2): 113–116. doi:10.1021/np960028h.
- ^ Kaneko, Hajime (1971). "Aroma of cigar tobacco. II. Isolation of norambreinolide from cigar tobacco". Agricultural and Biological Chemistry. 35 (9): 1461–1462.
- ^ Jasiński M; Stukkens Y; Degand H; Purnelle B; Marchand-Brynaert J; Boutry M (2001). "A Plant Plasma Membrane ATP Binding Cassette–Type Transporter Is Involved in Antifungal Terpenoid Secretion". Plant Cell. 13 (5): 1095–107. doi:10.1105/tpc.13.5.1095. PMC 135550. PMID 11340184.
