Shuttle catalysis
Shuttle catalysis is used to describe catalytic reactions where a chemical entity of a donor molecule is transferred to an acceptor molecule.[1] In these reactions, while the number of chemical bonds of each reactant changes, the types and total number of chemical bonds remain constant over the course of the reaction. In contrast to many organic reactions which exothermicity practically renders them irreversible, reactions operated under shuttle catalysis are often reversible.[2] However, the position of the equilibrium can be driven to the product side through Le Chatelier’s principle. The driving forces for this equilibrium shift are typically the formation of a gas/precipitation, the use of high ground-state energy reactants or the formation of stabilized products or the excess equivalents of a reactant.[3]

The relocation of shuttled entities is often mediated by a transition metal catalyst, which serves to functionalize or defunctionalize a compound of interest. An advantage to this process is that it excludes the process of handling toxic or reactive raw chemical entities. However, these reactions require the development of catalytic systems that can efficiently deliver the shuttled entities between the reactants under mild conditions through a sequence of elementary steps.
Applications
[edit]Transfer hydrogenation
[edit]Transfer hydrogenation has been extensively studied to reduce various functional groups without requiring hazardous pressurized H2.[4]
Transfer hydroacylation
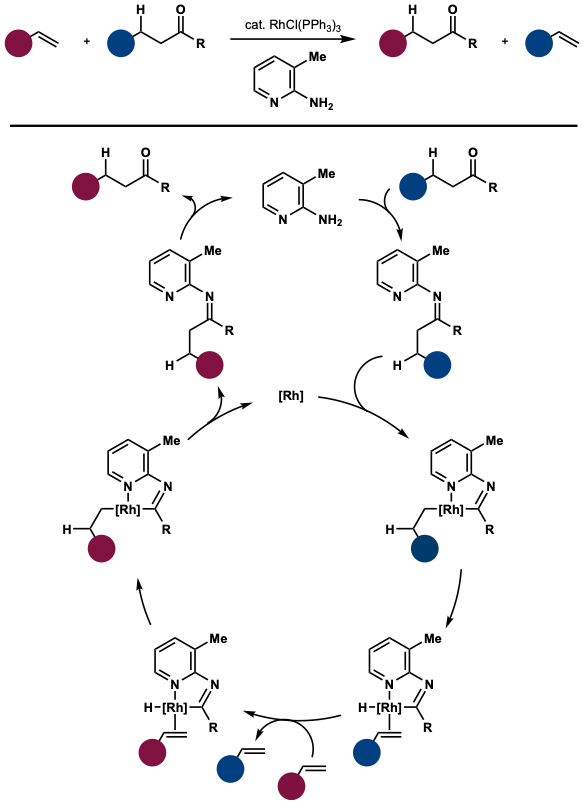
[edit]In 1999, Chul-Ho Jun and Hyuk Lee reported the first example of hydroacylation through shuttle catalysis.[5] In this example, 3-methyl-2-aminopyridine was used to activate the acyl group as well as coordinate to the rhodium catalyst, promoting C–C bond cleavage to eventually enable aldehyde transfer from a ketone to an alkene. The driving force of this reaction is the excess presence of alkenes and the formation of stable styrenes/extrusion of volatile ethylene. This method doesn’t require the use of toxic and self-reacting aldehydes such as acetaldehyde in the traditional hydroacylation procedures.
Transfer hydroformylation
[edit]Hydroformylation is a classical transition-metal catalyzed reaction, and it has been widely employed in industrial settings. However, a drawback of this reaction is the requirement of the hazardous mixture of H2/CO. For that reason, a process to replace H2/CO gas with a non-hazardous aldehyde is sought after.
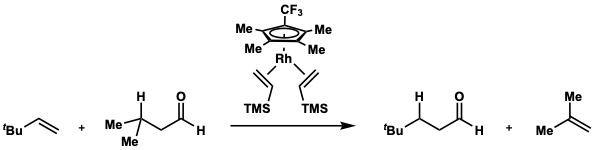
In 1999, Christian P. Lenges and Maurice Brookhart reported isovaleraldehyde as a suitable surrogate for H2/CO transfer to 3,3-dimethyl-1-butene by using a rhodium(I) catalyst.[6][7]
The reverse of this process was rendered catalytic by Vy M. Dong and co-workers in 2015.[8][9] They performed dehydroformylation on aldehydes to achieve the corresponding alkenes. For this transformation, they used either norbornene or norbornadiene as a H2/CO acceptor, promoting reactivity through strain-release.

Transfer hydrocyanation
[edit]To replace the use of toxic hydrocyanide (HCN) gas or surrogates such as acetone cyanohydrin, Bill Morandi and co-workers developed a hydrocyanation strategy using shuttle catalysis.[10] In this example, they use isovaleronitrile as a HCN surrogate under nickel/aluminum co-catalyzed conditions to afford hydrocyanation reactions of various alkenes. The use of isovaleronitrile allows careful control of HCN concentration, and the formation of volatile isobutylene is the driving force for the reaction.

Others
[edit]Other chemical entities including arenes,[11] CO/HCl,[12] HMgBr,[13] H2Zn,[14] H2O,[15] carbene,[16] silylene,[17] sulfenium[18] have also been shuttled under this catalysis platform.
References
[edit]- ^ Bhawal, Benjamin N.; Morandi, Bill (2017). "Shuttle Catalysis—New Strategies in Organic Synthesis". Chemistry – A European Journal. 23 (50): 12004–12013. doi:10.1002/chem.201605325. ISSN 1521-3765. PMID 28125163.
- ^ Bhawal, Benjamin N.; Morandi, Bill (2016-11-04). "Catalytic Transfer Functionalization through Shuttle Catalysis". ACS Catalysis. 6 (11): 7528–7535. doi:10.1021/acscatal.6b02333.
- ^ Bhawal, Benjamin N.; Morandi, Bill (2019). "Catalytic Isofunctional Reactions—Expanding the Repertoire of Shuttle and Metathesis Reactions". Angewandte Chemie International Edition. 58 (30): 10074–10103. doi:10.1002/anie.201803797. hdl:20.500.11850/320484. ISSN 1521-3773. PMID 30192427. S2CID 52169579.
- ^ Shimizu, Hideo; Nagasaki, Izuru; Matsumura, Kazuhiko; Sayo, Noboru; Saito, Takao (2007-12-01). "Developments in Asymmetric Hydrogenation from an Industrial Perspective". Accounts of Chemical Research. 40 (12): 1385–1393. doi:10.1021/ar700101x. ISSN 0001-4842. PMID 17685581.
- ^ Jun, Chul-Ho; Lee, Hyuk (1999-02-01). "Catalytic Carbon−Carbon Bond Activation of Unstrained Ketone by Soluble Transition-Metal Complex". Journal of the American Chemical Society. 121 (4): 880–881. doi:10.1021/ja983197s. ISSN 0002-7863.
- ^ Lenges, Christian P.; Brookhart, Maurice (1999). "Isomerization of Aldehydes Catalyzed by Rhodium(I) Olefin Complexes". Angewandte Chemie International Edition. 38 (23): 3533–3537. doi:10.1002/(SICI)1521-3773(19991203)38:23<3533::AID-ANIE3533>3.0.CO;2-E. ISSN 1521-3773. PMID 10602233.
- ^ Lenges, Christian P.; Brookhart, Maurice (1999). "Durch Rhodium(I)-Olefinkomplexe katalysierte Isomerisierung von Aldehyden". Angewandte Chemie (in German). 111 (23): 3746–3750. doi:10.1002/(SICI)1521-3757(19991203)111:23<3746::AID-ANGE3746>3.0.CO;2-B. ISSN 1521-3757.
- ^ "The Dong Research Group - About Professor Vy M. Dong". www.chem.uci.edu. Retrieved 2021-06-12.
- ^ Murphy, Stephen K.; Park, Jung-Woo; Cruz, Faben A.; Dong, Vy M. (2015-01-02). "Rh-catalyzed C–C bond cleavage by transfer hydroformylation". Science. 347 (6217): 56–60. doi:10.1126/science.1261232. ISSN 0036-8075. PMC 4445961. PMID 25554782.
- ^ Fang, Xianjie; Yu, Peng; Morandi, Bill (2016-02-19). "Catalytic reversible alkene-nitrile interconversion through controllable transfer hydrocyanation". Science. 351 (6275): 832–836. doi:10.1126/science.aae0427. ISSN 0036-8075. PMID 26912891. S2CID 29949044.
- ^ Lutz, Marius D. R.; Gasser, Valentina C. M.; Morandi, Bill (2021-04-08). "Shuttle arylation by Rh(I) catalyzed reversible carbon–carbon bond activation of unstrained alcohols". Chem. 7 (4): 1108–1119. doi:10.1016/j.chempr.2021.02.029. hdl:20.500.11850/476420. ISSN 2451-9294.
- ^ Fang, Xianjie; Cacherat, Bastien; Morandi, Bill (2017-11-05). "CO- and HCl-free synthesis of acid chlorides from unsaturated hydrocarbons via shuttle catalysis". Nature Chemistry. 9 (11): 1105–1109. doi:10.1038/nchem.2798. ISSN 1755-4349. PMID 29064496.
- ^ Bhawal, Benjamin N.; Morandi, Bill (2019). "Catalytic Isofunctional Reactions—Expanding the Repertoire of Shuttle and Metathesis Reactions". Angewandte Chemie International Edition. 58 (30): 10074–10103. doi:10.1002/anie.201803797. hdl:20.500.11850/320484. ISSN 1521-3773. PMID 30192427. S2CID 52169579.
- ^ Vettel, Stephan; Vaupel, Andrea; Knochel, Paul (1996-01-01). "Nickel-Catalyzed Preparations of Functionalized Organozincs". The Journal of Organic Chemistry. 61 (21): 7473–7481. doi:10.1021/jo960808v. ISSN 0022-3263. PMID 11667677.
- ^ Maffioli, Sonia I.; Marzorati, Ettore; Marazzi, Alessandra (2005-11-01). "Mild and Reversible Dehydration of Primary Amides with PdCl2 in Aqueous Acetonitrile". Organic Letters. 7 (23): 5237–5239. doi:10.1021/ol052100l. ISSN 1523-7060. PMID 16268547.
- ^ Gassman, Paul G.; Johnson, Thomas H. (1976-09-01). "Cyclopropane-olefin cross metathesis". Journal of the American Chemical Society. 98 (19): 6058–6059. doi:10.1021/ja00435a058. ISSN 0002-7863.
- ^ Ćiraković, Jelena; Driver, Tom G.; Woerpel, K. A. (2002-08-01). "Metal-Catalyzed Silacyclopropanation of Mono- and Disubstituted Alkenes". Journal of the American Chemical Society. 124 (32): 9370–9371. doi:10.1021/ja020566i. ISSN 0002-7863. PMID 12167021.
- ^ Adam, Waldemar; Bargon, Rainer M.; Schenk, Wolfdieter A. (2003-04-01). "Direct Episulfidation of Alkenes and Allenes with Elemental Sulfur and Thiiranes as Sulfur Sources, Catalyzed by Molybdenum Oxo Complexes". Journal of the American Chemical Society. 125 (13): 3871–3876. doi:10.1021/ja029292p. ISSN 0002-7863. PMID 12656621.
