Sulfoxide

A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides. (The use of the alternative spelling sulphoxide is discouraged by IUPAC.) An example of a sulfoxide occurring in nature is alliin.
Nature of the bond
Sulfoxides are generally represented with the structural formula R–S(=O)–R', where R and R' are organic groups. The bond between the sulfur and oxygen atoms is a dative bond and does not utilize the d-orbitals on sulfur as is commonly described in older textbooks. The S–O interaction has an electrostatic aspect, resulting in significant dipolar character, with negative charge centered on oxygen.

A lone pair of electrons resides on the sulfur atom giving it tetrahedral molecular geometry as for sp³ carbon. When the two organic residues are dissimilar, the sulfur is a chiral center, for example, methylphenylsulfoxide.
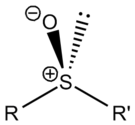
The energy required to invert this stereocenter is sufficiently high that sulfoxides are optically stable, that is, the rate of racemization is slow at room temperature. Chiral sulfoxides find application in certain drugs such as esomeprazole and Armodafinil, and they are also employed as chiral auxiliaries.[1] Many chiral sulfoxides are prepared from asymmetric catalytic oxidation of achiral sulfides with a transition metal and a chiral ligand.
Reactions
Sulfides are the usual starting materials to sulfoxides by organic oxidation. For example, dimethyl sulfide with oxidation state of -2 is oxidized to dimethyl sulfoxide with oxidation state 0. Further oxidation converts the compound to dimethyl sulfone wherein sulfur has the oxidation state +2. They are also excellent directing groups. [2]
Sulfoxides, such as DMSO, have basic character, being excellent ligands and readily alkylated. Alkyl sulfoxides are susceptible to deprotonation by strong bases, such as sodium hydride.[3]
References
- ^ Oxidation of sulfides to chiral sulfoxides using Schiff base-vanadium (IV) complexes Ángeles Gama, Lucía Z. Flores-López, Gerardo Aguirre, Miguel Parra-Hake, Lars H. Hellberg, and Ratnasamy Somanathan Arkivoc MX-789E 2003 Online article
- ^ http://www.chem.harvard.edu/groups/myers/handouts/10_Directed_Ortho.pdf
- ^ Iwai, I.; Ide, J. (1988). "2,3-Diphenyl-1,3-Butadiene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 531.
