Suosan
Appearance
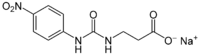
| |
| Names | |
|---|---|
| IUPAC name
Sodium N-[(4-nitrophenyl)carbamoyl]-β-alaninate
| |
| Systematic IUPAC name
Sodium 3-{[(4-nitrophenyl)carbamoyl]amino}propanoate | |
| Other names
N-(((4-Nitrophenyl)amino)carbonyl)-β-alanine monosodium salt
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C10H10N3NaO5 | |
| Molar mass | 275.196 g·mol−1 |
| Melting point | 240 °C (464 °F; 513 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Suosan is calorie-free artificial sweetener derived from β-alanine, discovered in 1948 by Petersen et Muller.[1]
Suosan is a sodium salt of p-Nitrophenylcarbamidopropionic acid and is 700 times sweeter than sucrose (table sugar)[2] with a bitter aftertaste.[3] It was never commercialized due to its low solubility in water, particularly under acidic pH (which limited its use, particularly in soft drinks)[4][5] and concerns that it might form the toxic compound 4-nitroaniline.[5]
See also
[edit]References
[edit]- ^ Petersen S; Muller E (1948). "Über eine neue Gruppe von Süsstoffen (On a new group of sweet substances)". Chemische Berichte. 81: 31–38. doi:10.1002/cber.19480810105.
- ^ Santhosh, C.; Mishra, P. C. (1994). "Electrostatic potential and electric field mapping of some sweeteners of the suosan series: A search for the structure-activity relationship". International Journal of Quantum Chemistry. 51 (5): 335. doi:10.1002/qua.560510510.
- ^ AD Kinghorn & CM Compadre (2001). "Less common high-potency sweeteners". In Marcel Dekker (ed.). Alternative Sweeteners (Third ed.). New York. pp. 208–234. ISBN 0-8247-0437-1.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Muller, George W; Culberson, J. Chris; Roy, Glenn; Ziegler, Jeanette; Walters, D. Eric; Kellogg, Michael S.; Schiffman, Susan S.; Warwick, Zoe S (May 1992). "Carboxylic acid replacement structure-activity relationships in suosan type sweeteners. A sweet taste antagonist. 1". J. Med. Chem. 35 (10): 1747–1751. doi:10.1021/jm00088a008. PMID 1588556.
- ^ a b Nofre, Claude; Tinti, Jean M; Chatzopoulos, Farroudja O (Mar 5, 1991). "Pyridinyl compounds of N-carbamoyl-N-thiocarbamoyl- or N-amidino-glycine or beta-alanine useful as sweetening agents. US Patent 4997667 A". Retrieved 14 September 2014.
External links
[edit] Media related to Suosan at Wikimedia Commons
Media related to Suosan at Wikimedia Commons
