Transurethral microwave thermotherapy
| Transurethral microwave thermotherapy | |
|---|---|
 Transurethral microwave thermotherapy catheter in situ | |
| ICD-9-CM | 60.96 |
| MeSH | D020728 |
Transurethral microwave thermotherapy (TUMT) is one of a number of effective and safe[1] procedures used in the treatment of lower urinary tract symptoms (LUTS) caused by benign prostatic hyperplasia (BPH). TUMT provides a one-time efficacious treatment of LUTS due to BPH. It is an alternative treatment to pharmacotherapy such as Alpha blockers, transurethral resection of the prostate (TURP), transurethral needle ablation of the prostate (TUNA), photoselective vaporization of the prostate (PVP) and prostatic removal or prostatectomy.[2]
Process
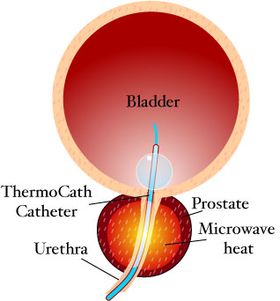
TUMT is a non-surgical, minimally invasive therapy that can be performed under a local anesthetic on an outpatient basis. The treatment involves inserting a special microwave urinary catheter into the hyperplastic prostatic urethra. The microwave antenna within the catheter then emits microwaves to heat and destroy the surrounding prostatic tissue.
The procedure can take from 30 minutes to one hour and is well tolerated by patients. Following the procedure, the prostatic tissue will be swollen and irritated. Urologists often place a Foley catheter to prevent the patient from having urinary retention. After 3 to 5 days the Foley can be replaced by a temporary prostatic stent to improve voiding without exacerbating irritation symptoms.[3]
Risks
The main risks of transurethral microwave thermotherapy (TUMT) include:
- Urinary retention
- Infection
- Postprocedural pain.
- Retrograde ejaculation
- Intense pain during the procedure
- Urination pain for several weeks
Evidence is turning in favour of TUMT over Transurethral resection of the prostate (TURP).[4]
Aftercare
Often the International Prostate Symptom Score (IPSS) including Quality of Life, is used to quantify symptoms and to monitor the response to the TUMT treatment. Fortunately post treatment convalescence is relatively rapid, with most patients able to void and a mean recovery time of less than 5 days at home.
However, prostatic edema is expected after microwave therapy, and this can lead to a risk of urinary retention. While some protocols suggest leaving a Foley catheter in for up to 2 weeks in all patients, other urologists are choosing to place a temporary prostatic stent after the first week following treatment. The stent is worn for 30 days and allows the patient to have volitional voiding with improved quality of life versus a Foley catheter. Generally urinary flow improves over a few months.
Patients maintained on alpha-blockers after TUMT may experience fewer urinary symptoms and have a decreased incidence of retention.[5]
See also
References
- ^ Stravodimos KG, Goldfischer ER, Klima WJ, Jabbour ME, Smith AD. 1998 Jun. Transurethral microwave thermotherapy for management of benign prostatic hyperplasia: a single-institution experience. | PMID 9609641
- ^ Jonathan Rubenstein, MD | Transurethral Microwave Thermotherapy of the Prostate (TUMT) http://www.emedicine.com/med/topic3070.htm | Feb 6, 2008
- ^ Dineen MK, Shore ND, Lumerman JH, Saslawsky MJ, Corica AP (2008 Mar 26). Use of a Temporary Prostatic Stent After Transurethral Microwave Thermotherapy Reduced Voiding Symptoms and Bother Without Exacerbating Irritative Symptoms. PMID 18374395
- ^ Rowland Illing (2007). "Surgical and Minimally Invasive Interventions for LUTS/BPH: Highlights from 2006". European Urology Supplements. 6 (12): 701–709. doi:10.1016/j.eursup.2007.04.003.
- ^ Neal D. Shore; Martin K. Dineenb‡; Mark J. Saslawskyc; Jeffrey H. Lumermand; Alberto P. Corica. (1999). "Prospective randomized comparison of high energy transurethral microwave thermotherapy versus alpha-blocker treatment of patients with benign prostatic hyperplasia". J. Urol. 161 (1): 139–43. doi:10.1016/S0022-5347(01)62084-6. PMID 10037386.
