Trimethylene carbonate
Appearance
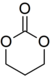
| |
| Names | |
|---|---|
| IUPAC name
1,3-Dioxan-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.114.239 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O3 | |
| Molar mass | 102.089 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trimethylene carbonate or 1,3-propylene carbonate is a 6-membered cyclic carbonate ester. It is a colourless solid that upon heating converts to the polytrimethyl carbonate. Such polymers are of interest for biomedical applications. An isomeric derivative is propylene carbonate, a colourless liquid that does not spontaneously polymerize.
Preparation
This compound may be prepared from 1,3-propanediol and ethyl chloroformate (a phosgene substitute), or from oxetane and carbon dioxide with an appropriate catalyst:[1]
- HOC3H6OH + ClCO2C2H5 → C3H6O2CO + C2H5OH + HCl
- C3H6O + CO2 → C3H6O2CO
This cyclic carbonate undergoes ring-opening polymerization to give poly(trimethylene carbonate), abbreviated PTC.[1] The polymer PTC is of commercial interest as a biodegradable polymer with biomedical applications.[2]
References
- ^ a b Pyo, Sang-Hyun; Persson, Per; Mollaahmad, M. Amin; Sörensen, Kent; Lundmark, Stefan; Hatti-Kaul, Rajni (2012). "Cyclic carbonates as monomers for phosgene- and isocyanate-free polyurethanes and polycarbonates". Pure Appl. Chem. 84 (3): 637. doi:10.1351/PAC-CON-11-06-14.
- ^ Engelberg, Israel; Kohn, Joachim (1991). "Physicomechanical properties of degradable polymers used in medical applications: a comparative study". Biomaterials. 12 (3): 292–304. doi:10.1016/0142-9612(91)90037-B.
