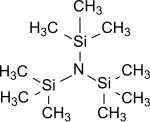
Tris(trimethylsilyl)amine
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,1-Trimethyl-N,N-bis(trimethylsilyl)silanamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.014.951 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H27NSi3 | |
| Molar mass | 233.57g/mol |
| Appearance | Waxy solid |
| Melting point | 67–69°C |
| Boiling point | 215°C (85°C at 13mmHg) |
| Solubility | Nonpolar organic solvents |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tris(trimethylsilyl)amine is the simplest tris(trialkylsilyl)amine which are having the general formula (R3Si)3N, in which all three hydrogen atoms of the ammonia are replaced by trimethylsilyl groups (-Si(CH3)3).[1] Tris(trimethylsilyl)amine has been for years in the center of scientific interest as a stable intermediate in chemical nitrogen fixation (i. e. the conversion of atmospheric nitrogen N2 into organic substrates under normal conditions).[2][3][4]
Production
[edit]Early attempts to prepare tris(trimethylsilyl)amine from ammonia and trimethylchlorosilane (TMS-Cl) were unsuccessful even at temperatures of 500 °C and in the presence of the base pyridine.[5][6] The reaction of ammonia and trimethylchlorosilane stops at the stage of the doubly silylated product bis(trimethylsilyl)amine (usually referred to as hexamethyldisilazane, HMDS).

Tris(trimethylsilyl)amine is obtained by reaction of the sodium salt of hexamethyldisilazane - from hexamethyldisilazane and sodium amide[7] or from hexamethyldisilazane, sodium and styrene[1] - with trimethylchlorosilane in 80% yield.[8]
The lithium salt of hexamethyldisilazane - from hexamethyldisilazane and butyllithium[9] or from hexamethyldisilazane and phenyllithium[8] - reacts with trimethylchlorosilane only in yields of 50-60% to tris(trimethylsilyl)amine.
The reaction of lithium nitride with trimethylchlorosilane can be carried out as a one-pot reaction in THF with 72% yield.[10]
Properties
[edit]Tris(trimethylsilyl)amine is a colorless, crystalline[11][12] or waxy[7] solid which is stable to water and bases.[13] Alcohols or acids though cleave the Si-N-bond under formation of ammonia.[7]
Applications
[edit]Tris(trimethylsilyl)amine as a synthetic building block
[edit]From antimony trichloride and tris(trimethylsilyl)amine, a nitridoantimone cubane-type cluster can be formed almost quantitatively at –60 °C.[14]
Ketones can be trifluoromethylated in the presence of P4-t-Bu and nonamethyltrisilazane under mild conditions in yields of up to 84% with the inert fluoroform (HCF3, HFC-23).[15]
The monomer trichloro(trimethylsilyl)-phosphoranimine Cl3P=NSiMe3 is formed from tris(trimethylsilyl)amine and phosphorus pentachloride in hexane at 0 °C,

which can be polymerized to linear polydichlorophosphazenes with defined molecular weights and polydispersities.[16]
The cyclic trimer (NPCl2)3 hexachlorocyclotriphosphane is predominantly formed from tris(trimethylsilyl)amine and phosphorus pentachloride in boiling dichloromethane (about 40 °C) among other oligomers which gives upon heating over 250 °C high molecular weight, little defined polydichlorophosphazenes.
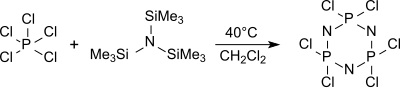
Nitrogen trifluoride NF3 (which is used, inter alia, for the plasma etching of silicon wafers) is obtainable from tris(trimethylsilyl)amine and fluorine at –40 °C in acetonitrile, suppressing the formation of nitrogen and tetrafluorohydrazine, which are produced as undesirable by-products during the standard synthesis of nitrogen trifluoride from ammonia or ammonium fluoride.[17]

Tris(trimethylsilyl)amine intermediate in chemical nitrogen fixation
[edit]The technical nitrogen fixation was made possible by the Haber-Bosch process, in which nitrogen is converted into ammonia by reductive protonation in the presence of iron catalysts under high pressures (> 150 bar) and temperatures (> 400 °C). In chemical nitrogen fixation (i.e., the transformation of atmospheric nitrogen under normal conditions into reactive starting materials for chemical syntheses, usually also ammonia), tris(trimethylsilyl)amine plays an important role in the so-called reductive silylation, since it is hydrolyzed with water to ammonia.
As early as 1895 it was observed that metallic lithium reacts with nitrogen to lithium nitride at room temperature.[18] In 1972, K. Shiina observed that lithium (as an electron donor) forms with trimethylsilyl chloride under darkening tris(trimethylsilyl)amine in the presence of chromium(III) chloride as a catalyst at room temperature with the nitrogen used for inerting.[2]
More recently, for the reductive silylation of N2, sodium has been used instead of lithium as the electron donor and molybdenum[19] and iron compounds[3] (such as pentacarbonyl iron or ferrocenes[20]) as catalysts, up to 34 equivalents of N(Me3Si)3 could be obtained per iron atom in the catalyst.
With a molybdenum-ferrocene complex as catalyst, a turnover number of up to 226 could be achieved.[21]
The catalytic productivity of the catalysts for chemical nitrogen fixation developed so far is, despite intensive research,[22] still by magnitude smaller than, for example, the modern polymerization catalysts of the metallocene type or enzymes.
References
[edit]- ^ a b J. Goubeau, J. Jiminéz-Barberá (1960), "Tris-(trimethylsilyl)-amin", ZAAC (in German), vol. 303, no. 5–6, pp. 217–226, doi:10.1002/zaac.19603030502
- ^ a b K. Shiina (1972), "Reductive silylation of molecular nitrogen via fixation to tris(trimethylsilyl)amine", J. Am. Chem. Soc., vol. 94, no. 26, pp. 9266–9267, doi:10.1021/ja00781a068
- ^ a b K.C. MacLeod, P.L. Holland (2013), "Recent developments in the homogeneous reduction of dinitrogen by molybdenum and iron", Nature Chemistry, vol. 5, no. 7, pp. 559–565, Bibcode:2013NatCh...5..559M, doi:10.1038/nchem.1620, PMC 3868624, PMID 23787744
- ^ W.I. Dzik (2016), "Silylation of dinitrogen catalyzed by hydridodinitrogen(triphenylphosphine) cobalt (I)", Inorganics, vol. 4, no. 3, p. 21, doi:10.3390/inorganics4030021
- ^ R.O. Sauer (1944), "Derivatives of the methylchlorosilanes. I. Trimethylsilanol and its simple ethers", J. Am. Chem. Soc., vol. 66, no. 10, pp. 1707–1710, doi:10.1021/ja01238a030
- ^ R.O. Sauer, R.H. Hasek (1946), "Derivatives of the methylchlorosilanes. IV. Amines", J. Am. Chem. Soc., vol. 68, no. 2, pp. 241–244, doi:10.1021/ja01206a028
- ^ a b c C.R. Krüger, H. Niederprüm, M. Schmidt, O. Scherer (1966), H.F. Holtzlow (ed.), Sodium Bis(trimethylsilyl)amide and Tris(trimethylsilyl)amine, in Inorganic Syntheses, vol. 8, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 15–19, doi:10.1002/9780470132395.ch5, ISBN 9780470131671
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b U. Wannagat, H. Niederprüm (1961), "Beiträge zur Chemie der Silicium-Stickstoff-Verbindungen, XIII. Silylsubstituierte Alkaliamide", Chem. Ber. (in German), vol. 94, no. 6, pp. 1540–1547, doi:10.1002/cber.19610940618
- ^ E.H. Amonoo-Neizer, R.A. Shaw, D.O. Skovlin, B.C. Smith, J.W. Rosenthal, W.L. Jolly (1966), H.F. Holtzlow (ed.), Lithium Bis(trimethylsilyl)amide and Tris(trimethylsilyl)amine, in Inorganic Syntheses, vol. 8, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 19–22, doi:10.1002/9780470132395.ch5, ISBN 9780470131671
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ W.L. Lehn (1964), "Preparation of tris(trimethylsilyl)- and tris(trimethylstannyl)amines", J. Am. Chem. Soc., vol. 86, no. 2, p. 305, doi:10.1021/ja01056a057
- ^ Sigma-Aldrich Co., product no. {{{id}}}.
- ^ Nonamethyltrisilazane at AlfaAesar, accessed on 28. Dezember 2016 (PDF) (JavaScript required).[dead link]
- ^ U. Wannagat, H. Niederprüm (1961), "dreifach silylierte Amine", ZAAC (in German), vol. 308, no. 1–6, pp. 337–351, doi:10.1002/zaac.19613080135
- ^ M. Rhiel, F. Weller, J. Pebler, K. Dehnicke (1994), "[SbN(SbCl)3(NSbCl2)(NSiMe3)3·SbCl3], ein ungewöhnlicher Nitridoantimonkomplex mit Heterocubanstruktur", Angew. Chem. (in German), vol. 106, no. 5, pp. 599–600, Bibcode:1994AngCh.106..599R, doi:10.1002/ange.19941060519
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ S. Okusu, K. Hirano, E. Tokunaga, N. Shibata (2015), "Organocatalyzed trifluormethylation of ketones and sulfonyl fluorides by fluoroform under a superbase system", ChemistryOpen, vol. 4, no. 5, pp. 581–585, doi:10.1002/open.201500160, PMC 4608523, PMID 26491635
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ US 5698664, H.R. Allcock, C.T. Morrissey, I. Manners, C.H. Honeyman, "Synthesis of polyphosphazenes with controlled molecular weight and polydispersity", published 1997-12-16, assigned to The Penn State Research Foundation, University of Toronto
- ^ US 8163262, B. A. Omotowa, "Method for production of nitrogen fluoride from trimethylsilylamines", published 2012-4-24
- ^ H. Deslandres (1895), "Absorption de l'azote par le lithium à froid", Comptes rendus, vol. 121, pp. 886–887
- ^ Q. Liao, N. Saffon-Merceron, N. Mézailles (2015), "N2 reduction into silylamine at tridentate phosphine/Mo center: catalysis and mechanistic study", ACS Catal., vol. 5, no. 11, pp. 6902–6906, doi:10.1021/acscatal.5b01626
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ M. Yuki, H. Tanaka, K. Sasaki, Y. Miyake, K. Yoshizawa, Y. Nishibayashi (2012), "Iron-catalyzed transformation of molecular dinitrogen into silylamine under ambient conditions", Nature Communications, vol. 3, p. 1254, Bibcode:2012NatCo...3.1254Y, doi:10.1038/ncomms2264, PMID 23212383
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ H. Tanaka; et al. (2011), "Molybdenum-Catalyzed Transformation of Molecular Dinitrogen into Silylamine: Experimental and DFT Study on the Remarkable Role of Ferrocenyldiphosphine Ligands", J. Am. Chem. Soc., vol. 133, no. 10, pp. 3498–3506, doi:10.1021/ja109181n, PMID 21341772
- ^ Y. Nishibayashi (2015), "Recent progress in transition-metal-catalyzed reduction of molecular dinitrogen under ambient reaction conditions", Inorg. Chem., vol. 54, no. 19, pp. 9234–9247, doi:10.1021/acs.inorgchem.5b00881, PMID 26131967

![{\displaystyle {\begin{matrix}{}\\{\ce {[(CH3)3Si]2NH ->[+{\ce {NaNH2}}][-{\ce {NH3}}] NaN[Si(CH3)3]2 ->[+{\ce {ClSi(CH3)3}}][-{\ce {NaCl}}] N[Si(CH3)3]3}}\\{}\end{matrix}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/78549476135b927dc95d6c81bc50de9b89e0b7ae)

![{\displaystyle {\ce {{N2}+{6e^{-}}->[{\ce {Catalyst:}}\ {\ce {Mo}},\ {\ce {Fe}},\ {\ce {Co}}]}}{\begin{cases}{\ce {->[{\ce {H+}}]}}&{\ce {2NH3}}\\{}\\{\ce {->[{\ce {R3Si-X}}][-\,{\ce {X-}}]}}&{\ce {2N(SiR3)3}}\end{cases}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7aa5ae0b13d71a31c97f22297303dee5ed50221a)
![{\displaystyle {\ce {N2 + 6Me3SiCl + 6}}\,{\color {NavyBlue}{\ce {Li}}}\ {\ce {->[{\ce {CrCl3}}] 2N(SiMe3)3 + 6}}\,{\color {NavyBlue}{\ce {Li}}}{\ce {Cl}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b7e5556a07b9c108214c4261b7694524d5af5665)
![{\displaystyle {\ce {N2 + 6Me3SiCl + 6}}\,{\color {Red}{\ce {Na}}}\ {\ce {->[{\ce {Fe-catalyst}}] 2N(SiMe3)3 + 6}}\,{\color {Red}{\ce {Na}}}{\ce {Cl}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ecf55c39b3edb4a67900c2fb80e508b6a1b31d91)
![{\displaystyle {\color {Red}{\ce {N2}}}+{\color {NavyBlue}{\ce {Me3Si}}}{\ce {{Cl}+Na->[{\ce {Mo/Fe-catalyst}}.][{\ce {RT}} \atop (1\ {\ce {atm}})]}}\ {\color {Red}{\ce {N}}}{\color {NavyBlue}{\ce {(Me3Si)3}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6e5a412bc2fab4fa67528a1779b7c318068be6f7)