User:Chem507f096/sandbox
16 Electron Complexes[edit]
Background[edit]
18 Electron Rule[edit]
Period 2 elements are stable when they possess the electronic configuration of neon (i.e. when they have a full octet). Similarly, transition metals are generally stable when they have the electronic configuration of argon.
In contrast to the usual octet rule for “simple” compounds, transition metals follow the 18-electron rule. Metals have 9 valence orbitals (1 s, 3 p, and 5 d), and to fill these orbitals 18 electrons are required. [1]
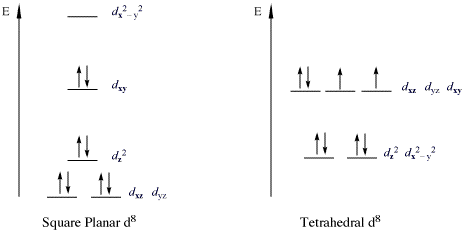
In general, 18-electron complexes do not present great catalytic activity compared to non-18 electron complexes (16, 17, 19, 20-electron complexes). Metal complexes of these kind are often key reactive intermediates in wide variety of chemical transformations, including reactions involving the activation of otherwise robust bonds in both organic and inorganic substrates.[2] The 18 electron rule is not universally obeyed, and several notable exceptions exist. In square planar d8 (ie. there are 8 d electrons on the metal) complexes, the 5 d-orbitals on the metal are not degenerate, and the dx2-y2 orbital is higher in energy, is not involved in bonding and is empty. As a result of this change in field splitting, the complexes 16 electrons are able to adequately stabilize the metal center. This is known as the 16 electron rule.
Square Planar vs. Tetrahedral Geometries[edit]
Most 16 electron transition metal complexes are tetracoordinate, and can either adopt a tetrahedral molecular geometry or square planar molecular geometry. The square planar molecular geometry is most common, due to ligand field stablization. Example of d8 square planar complexes involving Ni(II), Pd(II) and Pt(II) abound in organometallic catalysis. Tetrahedral complexes are more likely for non-transition metals or transition metals with no ligand field stabilization (d10, high-spin d5).
Synthesis[edit]
The literature, both past and present, is replete with examples of stable 16-electron complexes involving a variety different metals. The Sanford Group, for instance, reported the synthesis of a Benzquinoline Palladium(II) dimer with acetate bridging ligands. Each metal center in the dimer have square planar geometry, and electron counts of 16.[3]
Two famous 16-electron complexes are Vaska’s Complex and Wilkinson's catalyst. Wilkinson's catalyst can be prepared by refluxing RhCl3(H2O)3 and triphenyl phosphine (PPh3) in ethanol. [4]

Vaska’s Complex is synthesized starting with an Iridium Chloride complex, triphenyl phosphine and a carbon monoxide source.[5]

Fundamental Reactions[edit]
The reactions that involve 16 electron complexes can be classified into two categories: 1) Reactions in which 16 electron complexes are used as precursors. 2) Reactions in which 16 electron complexes exist as intermediates. According to the 16 and 18-Electron Rules, Lewis base ligand association, oxidative addition and deinsertion reactions (Table 1) can occur only with 16 electron complexes. Similarly, lewis base ligand dissociation, reductive elimination and insertion reactions are restricted to 18 electron complexes (Table 2), and involve 16 electron intermediates.[6][7]
Table 1:
| Rxn of 16-Electron Complex | Change in Valence Electron Count | Change in Oxidation State | Change in Coordination Number |
|---|---|---|---|
| Lewis Base Ligand Association | +2 | 0 | +1 |
| Oxidative Addition | +2 | +2 | +2 |
| Deinsertion | +2 | 0 | +1 |
Table 2:
| Rxn of 18-Electron Complex | Change in Valence Electron Count | Change in Oxidation State | Change in Coordination Number |
|---|---|---|---|
| Lewis Base Ligand Dissociation | -2 | 0 | -1 |
| Reductive Elimination | -2 | -2 | -2 |
| Insertion | -2 | 0 | -1 |
Lewis Base Association/Dissociation[edit]
The ligand exchange reaction for 16 electron complexes proceed via an association/dissociation mechanism (1), while 18 electron complexes undergo ligand exchange through a dissociation/association mechanism (2).[6]
Oxidative Addition/Reductive Elimination[edit]
The Iridium complex is known to undergo an oxidative addition with Hydrogen gas (3)[8]. In equation (4) the 16-electron Iridium intermediate is formed from the reductive elimination of Hydrogen gas from the original complex. This intermediate was isolable and has been reported.[9]
Insertion/Deinsertion[edit]
Equations (5) and (6) are examples of deinsertion at Manganese and insertion at Platinum.[6]
Hydroformylation Catalytic Cycle[edit]
In a day when reducing waste is a necessity, there is an emphasis on green chemistry and atom economy. Hydroformylation is an atom efficient process by which an alkene is converted to an aldehyde. Tollman and coworkers reported a hydroformylation using a Cobalt catalyst which alternates between 16 and 18 electrons throughout the cycle.[6] This particular catalytic cycle involves several fundamental reactions mentioned above.

- The catalytic cycle starts with Lewis base dissociation from the 18 electron complex (1) to give HCo(CO)3.
- The second step (2) involves Lewis base association of 16 electron complex followed by insertion of the olefin (3).
- These steps are repeated: Lewis base association (4) followed by an insertion (5).
- Step (6) is the oxidative addition of H2 followed by reductive elimination (7) of aldehyde to regenerate HCo(CO)3.
- Step (8) is the inhibition of hydroformylation by high CO pressure
References[edit]
- ^ Douglas, B., McDaniel, D., Alexander, J., Concepts and Models of Inorganic Chemistry. John Wiley & Sons, New York, 1994. ISBN 9780471305835.
- ^ Rankin M. A.; Schatte G.; McDonald R.; Stradiotto M. (2007). "Remarkably Facile and Reversible Ru-C(sp3) Bond Cleavage to Give a Reactive 16-Electron Cp*Ru(K2-P,Carbene) Zwitterion". J. Am. Chem. Soc.: 6390–6391. doi:10.1021/ja071684e.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hull K. L.; Sanford M. S. (2009). "Mechanism of Benzoquinone-Promoted Palladium-Catalyzed Oxidative Cross-Coupling Reactions". J. Am. Chem. Soc.: 1951–1953. doi:10.1021/ja901952h.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Osborn, J. A.; Jardine, F. H.; Young, J. F.; Wilkinson, G. (1966). "The Preparation and Properties of Tris(triphenylphosphine)halogenorhodium(I) and Some Reactions Thereof Including Catalytic Homogeneous Hydrogenation of Olefins and Acetylenes and Their Derivatives". J. Chem. Soc. A.: 1711–1732. doi:10.1039/J19660001711.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Girolami, G.S.; Rauchfuss, T.B.; Angelici, R.J. Synthesis and Technique in Inorganic Chemistry, Third ed.; University Science Books.: Sausalito, 1999, pp190. ISBN 0935702482.
- ^ a b c d Tolman C. A. (1972). "The 16 and 18 electron rule in Organometallic Chemistry and Homogeneous Catalysis". Chem. Soc. Rev.: 337–353. doi:10.1039/CS9720100337. Cite error: The named reference "number3" was defined multiple times with different content (see the help page).
- ^ Muetterties, E. L., Transition Metal Hydrides. Marcel Dekker Inc: New York, 1971. ISBN 9780824714703.
- ^ Chock P. B.; Halpern J. (1966). "Kinetics of the Addition of Hydrogen, Oxygen, and Methyl Iodide to Some Square-Planar Iridium (I) Complexes". J. Am. Chem. Soc.: 3511–3514. doi:10.1021/JA00967A009.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mays M. J.; Simpson R. N. F.; Stefanini F. P. (1970). "Cationic Complexes of Iridium. Part II. Kinetic Studies on the Reductive Elimination of Hydrogen from [IrH2(CO)2L2] (L=tertiary phosphine or tertiary arsine)". J. Am. Chem. Soc.: 3000. doi:10.1039/J19700003000.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
