User:Daniel duesentrieb/sandbox
| |
| Names | |
|---|---|
| IUPAC name
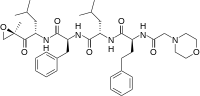
(S)-4-Methyl-N-((S)-1-(((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamide
| |
| Other names
PX-171-007
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C40H57N5O7 | |
| Molar mass | 719.924 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Carfilzomib (CFZ) is a tetrapeptide epoxyketone and a selective proteasome inhibitor. It is an analog of epoxomicin.[1] Unlike many other peptide drugs, Carfilzomib withstands gut passage and can be taken orally.
Discovery, early development and regulatory approval
[edit]Carfilzomib is derived from epoxomicin, a natural product that was shown by the laboratory of Craig Crews at Yale University to inhibit the proteasome.[2] The Crews laboratory subsequently invented a more specific derivative of epoxomicin named YU101,[3] which was licensed to Proteolix, Inc.. Scientists at Proteolix modified YU101 to create carfilzomib, which they advanced to multiple Phase 1 and 2 clinical trials, including a pivotal Phase 2 clinical trial designed to seek accelerated approval. Clinical trials for carfilzomib continue under Onyx Pharmaceuticals, which acquired Proteolix in 2009. In January 2011, the U.S. FDA granted carfilzomib fast-track status, allowing Onyx to initiate a rolling submission of its new drug application for carfilzomib.[4] In December 2011, the FDA granted Onyx standard review designation, [5][6] for its new drug application submission based on the 003-A1 study, an open-label, single-arm Phase 2b trial. The trial evaluated 266 heavily-pretreated patients with relapsed and refractory multiple myeloma who had received at least two prior therapies, including bortezomib and either thalidomide or lenalidomide.[7] Carfilzomib was approved by the FDA for use in patients with relapsed and refractory multiple myeloma on 20 July 2012.[8] Onyx expects to launch the drug in the U.S. on 1 August 2012. When it launches, it will cost $10,000 per 28-day cycle, making it the most expensive FDA-approved drug for multiple myeloma.[9]
Mechanism
[edit]Carfilzomib irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S proteasome, an enzyme that degrades unwanted cellular proteins. Inhibition of proteasome-mediated proteolysis results in a build-up of polyubiquinated proteins, which may cause cell cycle arrest, apoptosis, and inhibition of tumor growth.[1]
Clinical trials
[edit]A phase 2 trial for multiple myeloma showed promising results.[10][11]
A single-arm, phase 2 trial of carfilzomib in patients with relapsed and refractory multiple myeloma showed that single-agent carfilzomib had durable responses in 36 percent of the 257 patients evaluated.[12][13]
In another phase 2 trial of patients with relapsed and/or refractory multiple myeloma, carfilzomib in combination with lenalidomide and dexamethasone demonstrated an overall response rate of 78 percent. Researchers found carfilzomib could be administered over a period of 14–23 months with no new or overlapping toxicities.[13][14]
In a phase 2 trial, carfilzomib had a 53 percent overall response rate among patients with relapsed and/or refractory multiple myeloma who had not previously received bortezomib. This study also found prolonged carfilzomib treatment is well-tolerated with approximately 22 percent of patients continuing treatment beyond one year.[15]
In phase 2 trials of carfilzomib, the most common grade 3 or higher treatment-emergent adverse events were thrombocytopenia, anemia, lymphoenia, neutropenia, pneumonia, fatigue and hyponatremia.[16]
A phase 3 trial comparing carfilzomib, lenalidomide and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma is ongoing.[17]
In a frontline phase 1/2 study, the combination of carfilzomib, lenalidomide, and low-dose dexamethasone was highly active and well tolerated, permitting the use of full doses for an extended time in newly-diagnosed multiple myeloma patients, with limited need for dose modification. Responses were rapid and improved over time, reaching 100% very good partial response.[18]
References
[edit]- ^ a b Carfilzomib, NCI Drug Dictionary
- ^ Meng, L (1999). "Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity". Proc Natl Acad Sci USA. 96 (18): 10403–8. PMID 10468620.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Myung, J (2001). "Lack of proteasome active site allostery as revealed by subunit-specific inhibitors". Mol Cell. 7 (2): 411–20. PMID 11239469.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Onyx multiple myeloma drug wins FDA fast-track status". San Francisco Business Times. 2011-01-31. Retrieved 2011-09-01.
- ^ "Beacon Breaking News – Carfilzomib to Get Standard, Not Priority, FDA Review". The Myeloma Beacon. Retrieved 2012-02-27.
- ^ "Fast Track, Accelerated Approval and Priority Review; Accelerating Availability of New Drugs for Patients with Serious Diseases". FDA. Retrieved 2012-02-27.
- ^ "PX-171-003-A1, an open-label, single-arm, phase (Ph) II study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (R/R MM): Long-term follow-up and subgroup analysis". ASCO 2009.
{{cite web}}:|access-date=requires|url=(help); Missing or empty|url=(help); Unknown parameter|http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=ignored (help) - ^ "FDA approves Kyprolis for some patients with multiple myeloma". U.S. Food and Drug Administration.
- ^ "FDA Approves Kyprolis (Carfilzomib) For Relapsed And Refractory Multiple Myeloma". The Myeloma Beacon. Retrieved 2012-07-20.
- ^ Onyx Says Carfilzomib Results Promising, Drug Discovery & Development, July 27, 2010
- ^ "Phase II results of Study PX-171-007: A phase Ib/II study of carfilzomib (CFZ), a selective proteasome inhibitor, in patients with selected advanced metastatic solid tumors" - ASCO 2009; Abstract 3515.
- ^ "ASCO Showcasing Bay Area Cancer Therapies". San Francisco Business Times. 2011-06-02. Retrieved 2011-09-01.
- ^ a b "PX-171-003-A1, an open-label, single-arm, phase (Ph) II study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (R/R MM): Long-term follow-up and subgroup analysis". ASCO 2011; Abstract 8027. 2011. Retrieved 2011-09-01.
- ^ "Interim results from PX-171-006, a phase (Ph) II multicenter dose-expansion study of carfilzomib (CFZ), lenalidomide (LEN), and low-dose dexamethasone (loDex) in relapsed and/or refractory multiple myeloma (R/R MM)". ASCO 2011; Abstract 8025. 2011. Retrieved 2011-09-01.
- ^ "The effect of carfilzomib (CFZ) in patients (Pts) with bortezomib (BTZ)-naive relapsed or refractory multiple myeloma (MM): Updated results from the PX-171-004 study". ASCO 2011; Abstract 8026. 2011. Retrieved 2011-09-01.
- ^ "Siegel DS, Martin T, Wang, M, et al. Results of PX-171- 003-A1, an open-label, single-arm, phase 2 study of carfilzomib in patients with relapsed and refractory multiple myeloma. Presented at: 52nd ASH Annual Meeting and Exposition; December 4-7, 2010; Orlando, Florida". OncLive.com. 2011-03-09. Retrieved 2011-09-01.
- ^ "Phase 3 Study Comparing Carfilzomib, Lenalidomide, and Dexamethasone (CRd) Versus Lenalidomide and Dexamethasone (Rd) in Subjects With Relapsed Multiple Myeloma". ClinicalTrials.gov. 2011-08-04. Retrieved 2011-09-01.
- ^ "Final Results of a Frontline Phase 1/2 Study of Carfilzomib Lenalidomide, and Low-Dose Dexamethasone (CRd) in Multiple Myeloma (MM)". ASH 20111; Abstract 631. Retrieved 2012-02-27.

