User:Eb.hoop2/sandbox
Carnot cycle
[edit]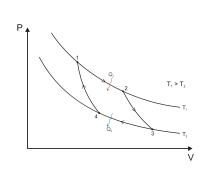
Carnot wanted to answer two questions about the operation of heat engines: "Is the work available from a heat source potentially unbounded?" and "Can heat engines in principle be improved by replacing the steam with some other working fluid or gas?" He attempted to answer these in a memoir that he wrote when he was only 27 years old and which was published in June of 1824, at the author's expense, in a printing of 600 copies. It was entitled Réflexions sur la puissance motrice du feu et sur les machines propres à développer cette puissance ("Reflections on the Motive Power of Fire and on Machines Fitted to Develop that Power").
That book was only 118 pages long and covered a wide range of topics about heat engines in what Carnot must have intended to be a form accessible to a wide public, with minimal use of mathematics (which he confined to elementary uses of algebra and arithmetic, except in some footnotes). Carnot discussed the relative merits of air and steam as working fluids, the merits of various aspects of steam-engine design, and even included some ideas of his own regarding possible practical improvements. However, the central part of the book was an abstract treatment of an idealized engine (the Carnot cycle) with which the author sought to clarify the fundamental principles that govern all heat engines, independently of the details of their design or operation. This resulted in an idealized thermodynamic system upon which exact calculations could be made, and avoided the complications introduced by many of the crude features of the contemporary steam engines.
The Carnot cycle is the most efficient possible engine, not only because of the (trivial) absence of friction, heat leakage, and other incidental wasteful processes; the main reason is that it works without heat ever flowing directly between bodies at different temperatures. Carnot knew that such heat conduction is a wasteful and irreversible process, which must be minimized if the net heat that passes from the hotter to the colder reservoir is to do the greatest possible amount of useful work.
The other key property of the Carnot cycle is that it is a reversible process: if the transformations involved in that process are carried out in reverse order, then work is used to transport heat from the colder back to the hotter reservoir. In Carnot's analysis, no "caloric" would be lost during the operation of this idealized refrigerator.

