User:Mr. Ibrahem/Adagrasib
Appearance
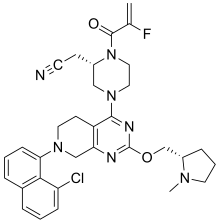 | |
| Clinical data | |
|---|---|
| Trade names | Krazati |
| Other names | MRTX-849 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth |
| Drug class | Antineoplastic agents |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C32H35ClFN7O2 |
| Molar mass | 604.13 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Adagrasib, sold under the brand name Krazati, is a medication used to treat non-small cell lung cancer (NSCLC).[3] Specifically it is used for KRAS G12C-mutated advanced cancer that has failed at list one other treatment.[3] It is taken by mouth.[3]
Common side effects include diarrhea, nausea, musculoskeletal pain, liver problems, kidney problems, shortness of breath, low potassium, low sodium, low white blood cells, and swelling.[3] Other side effects may include QT prolongation and pneumonitis.[3] It is an inhibitor of the RAS GTPase family.[3]
Adagrasib was approved for medical use in the United States in 2022.[3] In the United States it costs about 237,000 USD per year as of 2022.[4]
References[edit]
- ^ "Archive copy" (PDF). Archived (PDF) from the original on 2022-12-13. Retrieved 2022-12-18.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSC". U.S. Food and Drug Administration (FDA). 12 December 2022. Archived from the original on 14 December 2022. Retrieved 14 December 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f g h i j "DailyMed - KRAZATI- adagrasib tablet, coated". dailymed.nlm.nih.gov. Archived from the original on 14 January 2023. Retrieved 12 January 2023.
- ^ "FDA Approves Rescue Combination Medication for Asthma". Formulary Watch. Archived from the original on 14 January 2023. Retrieved 12 January 2023.
