User:Mr. Ibrahem/Amifampridine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Firdapse, Ruzurgi, Zenas |
| Other names | Amifampridine phosphate, pyridine-3,4-diamine, 3,4-diaminopyridine, 3,4-DAP |
| AHFS/Drugs.com | Monograph Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Potassium channel blocker[2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 93–100%[3] |
| Metabolism | Acetylation to 3-N-acetylamifampridine |
| Elimination half-life | 2.5 hrs (amifampridine) 4 hrs (3-N-acetylamifampridine) |
| Excretion | Kidney (19% unmetabolized, 74–81% 3-N-acetylamifampridine |
| Identifiers | |
| |
| Chemical and physical data | |
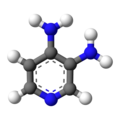
| Formula | C5H7N3 |
| Molar mass | 109.132 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 218 to 220 °C (424 to 428 °F) decomposes |
| Solubility in water | 24 mg/mL (20 °C) |
| |
| |
| | |
Amifampridine, sold under the brand name Firdapse and Ruzurgi, is a medication used to treat Lambert–Eaton myasthenic syndrome (LEMS).[4][2] It is taken by mouth.[5] There are two forms, one labelled for use in children and the other in adults.[6]
Common side effects include numbness, stomach pain, diarrhea, and nausea.[2] It should not be used in people with prolonged QT, epilepsy, or asthma.[2] There are concerns that use in pregnancy many harm the baby.[7] It is a potassium channel blocker which prolongs the time of nerve depolarization.[2]
Amifampridine was approved for medical use in Europe in 2009 and the United States in 2018.[2][4] In the United Kingdom 100 pills of 10 mg costs the NHS about £1,800 as of 2021.[5] This amount in the United States costs about 20,600 USD.[8]
References[edit]
- ^ a b "Ruzurgi". Therapeutic Goods Administration (TGA). 24 September 2021. Archived from the original on 30 September 2021. Retrieved 30 September 2021.
- ^ a b c d e f g "Firdapse (previously Zenas)". Archived from the original on 14 November 2021. Retrieved 14 January 2022.
- ^ "Firdapse Summary of Product Characteristics" (PDF). EMA. February 11, 2010. Archived (PDF) from the original on June 20, 2018. Retrieved September 30, 2021. See EMA Index page, product tab Archived 2018-09-20 at the Wayback Machine
- ^ a b c "DailyMed - RUZURGI- amifampridine tablet". dailymed.nlm.nih.gov. Archived from the original on 30 September 2021. Retrieved 14 January 2022.
- ^ a b BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1171. ISBN 978-0857114105.
- ^ "Amifampridine Phosphate Monograph for Professionals". Drugs.com. Archived from the original on 9 December 2020. Retrieved 14 January 2022.
- ^ "Amifampridine Use During Pregnancy". Drugs.com. Archived from the original on 23 November 2020. Retrieved 14 January 2022.
- ^ "Firdapse Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 26 January 2021. Retrieved 14 January 2022.
