User:Mr. Ibrahem/Benazepril
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /bəˈnæzəprɪl/ |
| Trade names | Lotensin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692011 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | ACE inhibitor[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 96.7% |
| Metabolism | Liver glucuronidation |
| Elimination half-life | 10 hours[2] |
| Excretion | Kidney and biliary |
| Identifiers | |
| |
| Chemical and physical data | |
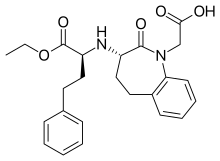
| Formula | C24H28N2O5 |
| Molar mass | 424.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Benazepril, sold under the brand name Lotensin among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] It is taken by mouth.[1] Versions are available as the combinations benazepril/hydrochlorothiazide and benazepril/amlodipine.[1]
Common side effects include feeling tired, dizziness, cough, and light-headedness with standing.[1] Serious side effects may include kidney problems, low blood pressure, high blood potassium, and angioedema.[1] Use in pregnancy may harm the baby while use when breastfeeding maybe okay.[4] It is an ACE inhibitor and works by decreasing renin-angiotensin-aldosterone system activity.[1]
Benazepril was patented in 1981 and came into medical use in 1990.[5] It is available as a generic medication.[1] A month supply in the United States costs about US$1.32 as of 2019.[6] In 2017, it was the 104th most commonly prescribed medication in the United States, with more than seven million prescriptions.[7][8]
References[edit]
- ^ a b c d e f g h i j k "Benazepril Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ Benowitz, Neal L. (2020). "11. Antihypertensive agents". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 183. ISBN 978-1-260-45231-0.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Retrieved 6 September 2020.
- ^ "Benazepril Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 468. ISBN 9783527607495.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- ^ "Benazepril Hydrochloride - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
