User:Mr. Ibrahem/Benzatropine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cogentin, others |
| Other names | Benztropine, benztropine (BAN UK), benztropine (USAN US) |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth, IM, IV |
| Drug class | Anticholinergic[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 12-24 hours |
| Excretion | Urine |
| Identifiers | |
| |
| Chemical and physical data | |
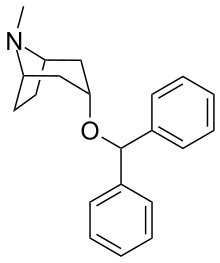
| Formula | C21H25NO |
| Molar mass | 307.437 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benzatropine, also spelled benztropine, is a medication used to treat a type of movement disorder due to antipsychotics known as dystonia and parkinsonism.[1] It is not useful for tardive dyskinesia.[1] It is taken by mouth or by injection into a vein or muscle.[1] Benefits are seen within two hours and last for up to ten hours.[3][4]
Common side effects include dry mouth, blurry vision, nausea, and constipation.[1] Serious side effect may include urinary retention, hallucinations, hyperthermia, and poor coordination.[1] It is unclear if use during pregnancy or breastfeeding is safe.[5] Benzatropine is an anticholinergic which works by blocking the activity of the muscarinic acetylcholine receptor.[1]
Benzatropine was approved for medical use in the United States in 1954.[1] It is available as a generic medication.[1] In the United States the wholesale cost is about US$6 per month.[6] In 2017, it was the 226th most commonly prescribed medication in the United States, with more than two million prescriptions.[7][8] It is sold under the brand name Cogentin among others.[1]
References[edit]
- ^ a b c d e f g h i j k l "Benztropine Mesylate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 June 2019. Retrieved 9 April 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 7 August 2020. Retrieved 7 September 2020.
- ^ Pagliaro, Louis A.; Pagliaro, Ann M. (1999). PNDR, Psychologists' Neuropsychotropic Drug Reference. Psychology Press. p. 47. ISBN 9780876309568. Archived from the original on 2020-07-26. Retrieved 2019-04-09.
- ^ Aschenbrenner, Diane S.; Venable, Samantha J. (2009). Drug Therapy in Nursing. Lippincott Williams & Wilkins. p. 197. ISBN 9780781765879. Archived from the original on 2020-07-26. Retrieved 2019-04-09.
- ^ "Benztropine (Cogentin) Use During Pregnancy". Drugs.com. Archived from the original on 6 June 2019. Retrieved 9 April 2019.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Benztropine Mesylate - Drug Usage Statistics". ClinCalc. Archived from the original on 3 July 2020. Retrieved 11 April 2020.
