User:Mr. Ibrahem/Chlortetracycline
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Aureomycin |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth, IV, topical |
| Drug class | Tetracycline[1] |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Protein binding | 50 to 55% |
| Metabolism | Gastrointestinal tract, liver (75%) |
| Metabolites | Isochlortetracycline |
| Elimination half-life | 5.6 to 9 hours |
| Excretion | 60% kidney and >10% biliary |
| Identifiers | |
| |
| Chemical and physical data | |
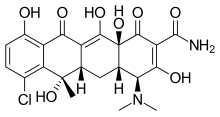
| Formula | C22H23ClN2O8 |
| Molar mass | 478.882 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | [α]D25−275°·cm3·dm−1·g−1 (methane) |
| Melting point | 168 to 169 °C (334 to 336 °F) |
| Solubility in water | 0.5–0.6 mg/mL (20 °C) |
| |
| |
| | |
Chlortetracycline, sold under the brand name Aureomycin among others, is an antibiotic used to treat conjunctivitis, including trachoma.[3] It is generally used as an eye drop.[5] It may also be used for Rickettsiae.[4]
Side effects may include tooth staining, sun sensitivity, nausea, and diarrhea.[4] Other side effects may include kidney and liver problems.[4] Use is not recommended during pregnancy or breastfeeding.[4] It is a tetracycline and works by interfering with protein production.[1]
Chlortetracycline was discovered in 1945 by Benjamin Minge Duggar and came into medical use in 1948.[6][7] The eye drop was approved in the United States in 1950; though is no longer commercially available.[5] It is on the World Health Organization's List of Essential Medicines as an alternative to tetracycline.[8] It is also used in veterinary medicine.[9]
References[edit]
- ^ a b Sneader, Walter (23 June 2005). Drug Discovery: A History. John Wiley & Sons. p. 304. ISBN 978-0-471-89979-2. Archived from the original on 13 September 2023. Retrieved 10 September 2023.
- ^ "chlortetracycline | C22H23ClN2O8 - PubChem". Pubchem.ncbi.nlm.nih.gov. Archived from the original on 2016-12-21. Retrieved 2017-03-13.
- ^ a b "eEML - Electronic Essential Medicines List". list.essentialmeds.org. Archived from the original on 29 May 2023. Retrieved 10 September 2023.
- ^ a b c d e Scholar, Eric (2007). "Chlortetracycline". xPharm: The Comprehensive Pharmacology Reference: 1–5. doi:10.1016/B978-008055232-3.61451-5.
- ^ a b "Chlortetracycline: Indications, Side Effects, Warnings". Drugs.com. Archived from the original on 12 August 2022. Retrieved 10 September 2023.
- ^ Ravina, Enrique (18 April 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 279. ISBN 978-3-527-32669-3. Archived from the original on 13 September 2023. Retrieved 10 September 2023.
- ^ Riviere, Jim E.; Papich, Mark G. (17 March 2009). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 902. ISBN 978-0-8138-2061-3. Archived from the original on 13 September 2023. Retrieved 10 September 2023.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Click to open content tools § 558.128 Chlortetracycline". Archived from the original on 20 March 2022. Retrieved 10 September 2023.
