User:Mr. Ibrahem/Cladribine
 | |
| Clinical data | |
|---|---|
| Trade names | Leustatin, Mavenclad, others[1] |
| Other names | 2-chlorodeoxyadenosine |
| AHFS/Drugs.com | Injectable: Monograph By mouth: Monograph |
| MedlinePlus | a693015 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, subcutaneous (liquid), by mouth (tablet) |
| Drug class | Antimetabolite (purine analogue)[2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (i.v.); 37 to 51% (by mouth)[3] |
| Protein binding | 25% (range 5-50%);[4] up to 20% (by mouth) [5] |
| Metabolism | Mostly via intracellular kinases; 15-18% is excreted unchanged[4]
IV and SQ bolus: 15-18% is excreted unchanged By mouth, 25% (±21%) of dose is excreted unchanged in urine and 3.8% as a metabolite[5] |
| Elimination half-life | ~10 hours IV and SQ[4] and 18.4 to 19.7 hours after by mouth |
| Excretion | Urinary[4] |
| Identifiers | |
| |
| Chemical and physical data | |
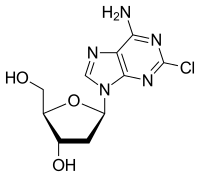
| Formula | C10H12ClN5O3 |
| Molar mass | 285.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cladribine, sold under the brand name Leustatin among others, is a medication used to treat hairy cell leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma.[6] It is also used for relapsing-remitting multiple sclerosis.[7] It is taken by mouth or by injection into a vein or under the skin.[7]
Common side effects include infection, anxiety, hair loss, arrythmias, diarrhea, fever, muscle pain, rash, and bleeding.[7] Other side effects may include bone marrow suppression, progressive multifocal encephalopathy (PML), tumor lysis syndrome, and nerve damage.[7] Use in pregnancy may harm the baby.[7] It is an antimetabolite, specifically a purine analogue, which interferes with the production of new DNA by lymphocytes.[2]
Cladribine has been in medical use since the 1980s, with use in parts of Europe since 1993.[2] It was also approved for medical use in the United States in 1993.[6] It is on the World Health Organization's List of Essential Medicines.[8] Some formulations are available as a generic medication.[6] In the United Kingdom 10 mg for injection costs about £160 while a 10 mg pill costs £2,050 as of 2021.[7] In the United States this amount costs about 370 USD and 9,000 USD respectively.[9][10]
References[edit]
- ^ "Cladribine". Drugs.com. 28 February 2020. Archived from the original on 4 March 2016. Retrieved 4 March 2020.
- ^ a b c "Litak". Archived from the original on 16 April 2021. Retrieved 5 January 2022.
- ^ Liliemark J (February 1997). "The clinical pharmacokinetics of cladribine". Clinical Pharmacokinetics. 32 (2): 120–31. doi:10.2165/00003088-199732020-00003. PMID 9068927. S2CID 32926069.
- ^ a b c d "PRODUCT INFORMATION LITAK© 2 mg/mL solution for injection" (PDF). TGA eBusiness Services. St Leonards, Australia: Orphan Australia Pty. Ltd. 10 May 2010. Archived from the original on 11 September 2016. Retrieved 27 November 2014.
- ^ a b Giovannoni, G (2017). "Cladribine to Treat Relapsing Forms of Multiple Sclerosis". Neurotherapeutics. 14 (4): 874–887. doi:10.1007/s13311-017-0573-4. PMC 5722776. PMID 29168160.
- ^ a b c d "Cladribine Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 5 January 2022.
- ^ a b c d e f g h BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 952. ISBN 978-0857114105.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Cladribine Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 April 2021. Retrieved 5 January 2022.
- ^ "Mavenclad Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 January 2021. Retrieved 5 January 2022.
