User:Mr. Ibrahem/Fostemsavir
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rukobia |
| Other names | Fostemsavir tromethamine, BMS-663068, GSK3684934 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620046 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | HIV fusion inhibitor[2] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
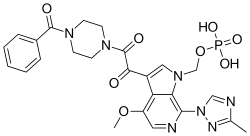
| Formula | C25H26N7O8P |
| Molar mass | 583.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fostemsavir, sold under the brand name Rukobia, is a medication used to treat HIV/AIDS.[4] It is used together with other medications, when usually treatments are not effective.[3] It is taken by mouth.[3]
Common side effects include nausea, diarrhea, rash, and abdominal pain.[3][4] Other side effects may include liver problems, QT prolongation, and immune reconstitution syndrome.[3] It should not be taken with strong CYP3A inducers.[4] It works by binding to the HIV virus and preventing it from entering T cells.[4]
Fostemsavir was approved for medical use in the United States 2020, and Europe in 2021.[2][4] In the United Kingdom a month of treatment costs the NHS £2,900 as of 2021.[5] In the United States this amount costs about 8,000 USD.[6]
References
[edit]- ^ a b "Rukobia". Therapeutic Goods Administration (TGA). 23 July 2021. Archived from the original on 5 September 2021. Retrieved 5 September 2021.
- ^ a b "Fostemsavir Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 12 December 2021.
- ^ a b c d e f g "Rukobia- fostemsavir tromethamine tablet, film coated, extended release". DailyMed. 2 July 2020. Archived from the original on 15 July 2020. Retrieved 14 July 2020.
- ^ a b c d e f g h "Rukobia EPAR". European Medicines Agency (EMA). 9 December 2020. Archived from the original on 12 February 2021. Retrieved 12 February 2021.
- ^ "Rukobia · HIV infection". Retrieved 13 December 2021.
{{cite web}}: CS1 maint: url-status (link) - ^ "Rukobia Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 13 December 2021.
