User:Mr. Ibrahem/Ganciclovir
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɡænˈsaɪkləvɪər/ |
| Trade names | Cytovene; Cymevene; Vitrasert; others |
| Other names | Ganciclovir sodium; gancyclovir; DHPG; 9-(1,3-dihydroxy-2-propoxymethyl)guanine |
| AHFS/Drugs.com | Systemic: Monograph Eye drop: Monograph |
| MedlinePlus | a605011 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, eye drop, by mouth, intravitreal |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5% (by mouth) |
| Metabolism | guanylate kinase (CMV UL97 gene product) |
| Elimination half-life | 2.5–5 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
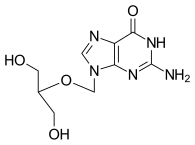
| Formula | C9H13N5O4 |
| Molar mass | 255.234 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 250 °C (482 °F) (dec.) |
| |
| |
| (verify) | |
Ganciclovir, sold under the brand name Cytovene among others, is an antiviral medication used to treat and prevent cytomegalovirus disease (CMV).[1] This includes CMV retinitis, pneumonitis, encephalitis, and congenital CMV disease.[3] It may also be used for certain VZV infections of the eye.[3] It is given by injection into a vein or the eye.[1] It may also be used as eye drops.[2]
Common side effects when given by injection include low white blood cells, low platelets, low red blood cells, fever, diarrhea, increased sweating, headache, and kidney problems.[3] Other side effects may include infertility and cancer.[3] Use in pregnancy may harm the baby.[3] It is a nucleoside analog of guanine.[3]
Ganciclovir was patented in 1980 and approved for medical use in 1988.[4] In the United Kingdom a 500 mg vial costs the NHS about £30 as of 2021.[1] This amount in the United States is about 70 USD.[5]
References[edit]
- ^ a b c d BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 676. ISBN 978-0857114105.
- ^ a b "Ganciclovir Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 2 December 2021.
- ^ a b c d e f g "Ganciclovir Sodium Monograph for Professionals". Drugs.com. Archived from the original on 4 May 2017. Retrieved 2 December 2021.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 504. ISBN 9783527607495. Archived from the original on 2016-12-20. Retrieved 2021-06-28.
- ^ "Ganciclovir Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 2 December 2021.
