User:Mr. Ibrahem/Lenacapavir
 | |
| Clinical data | |
|---|---|
| Trade names | Sunlenca |
| Other names | GS-CA1, GS-6207 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth, subcutaneous |
| Drug class | Capsid inhibitors[1] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
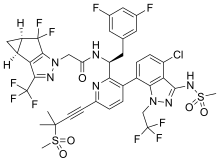
| Formula | C39H32ClF10N7O5S2 |
| Molar mass | 968.28 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lenacapavir, sold under the brand name Sunlenca, is an antiretroviral medication used to treat HIV/AIDS.[5] It is used together with other medications, in those with medication resistant disease.[1] It is started by mouth and than given by injection under the skin twice per year.[5]
Common side effects include reactions at the injection site and nausea.[5] Other side effects may include immune reconstitution syndrome.[1] While there is no evidence of harm in pregnacy, such use has not been well studied.[1] It is a capsid inhibitors and works by interfering with multiple steps of the viral lifecycle.[1]
Lenacapavir was approved for medical use in Europe, Canada, and the United States in 2022.[5][1][2] In the United States it costs about 42,250 USD the first year and 39,000 USD each year thereafter as of 2022.[6]
References[edit]
- ^ a b c d e f "DailyMed - SUNLENCA- lenacapavir sodium tablet, film coated SUNLENCA- lenacapavir sodium kit". dailymed.nlm.nih.gov. Archived from the original on 21 January 2023. Retrieved 1 February 2023.
- ^ a b "Sunlenca Product information". Health Canada. 25 April 2012. Archived from the original on 15 January 2023. Retrieved 23 December 2022.
- ^ "Sunlenca Product information". Health Canada. 25 April 2012. Archived from the original on 15 January 2023. Retrieved 23 December 2022.
- ^ "Archive copy" (PDF). Archived (PDF) from the original on 2023-01-15. Retrieved 2022-12-23.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ a b c d e f g "Sunlenca EPAR". European Medicines Agency (EMA). 22 June 2022. Archived from the original on 26 August 2022. Retrieved 25 August 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "FDA Approves First-in-Class Drug for HIV". Medscape. Archived from the original on 31 December 2022. Retrieved 1 February 2023.
