User:Mr. Ibrahem/Lutetium (177Lu) oxodotreotide
 | |
| Clinical data | |
|---|---|
| Trade names | Lutathera |
| Other names | Lutetium (177Lu) dotatate |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Intravenous |
| Drug class | Antineoplastic agent |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
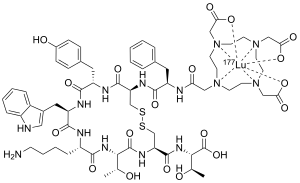
| Formula | C65H87LuN14O19S2 |
| Molar mass | 1607.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lutetium (177Lu) oxodotreotide, sold under the brand name Lutathera, is a medication used to treat gastroenteropancreatic neuroendocrine tumors (GEP-NET) which express somatostatin receptors.[4] It improved time with stable disease from 9 months to 28 months.[5] It is given by injection into a vein.[4]
Common side effects include low lymphocytes, liver inflammation, high blood sugar, low potassium, and nausea.[2] Other side effects may include low platelets, low red blood cells, and tiredness.[5] It works by attaching to somatostatin receptors after which the radioactivity gives the cell.[5]
Lutetium (177Lu) oxodotreotide was approved for medical use in Europe in 2017 and the United States in 2018.[5][4] In the United States it costs about 54,000 USD per dose as of 2021.[6]
References[edit]
- ^ "Lutathera 370 MBq/mL solution for infusion - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 9 July 2021. Retrieved 9 July 2021.
- ^ a b c "Lutathera- lutetium lu 177 dotatate injection". DailyMed. 4 May 2020. Archived from the original on 16 November 2020. Retrieved 8 November 2020.
- ^ "Lutathera EPAR". European Medicines Agency (EMA). Archived from the original on 11 December 2019. Retrieved 11 December 2019.
- ^ a b c d "Lutetium Lu 177 Dotatate Monograph for Professionals". Drugs.com. Archived from the original on 19 October 2021. Retrieved 24 November 2021.
- ^ a b c d "Lutathera". Archived from the original on 11 December 2019. Retrieved 24 November 2021.
- ^ "Lutathera Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 January 2021. Retrieved 24 November 2021.
