User:Mr. Ibrahem/Ponatinib
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /poʊˈnætɪnɪb/ poh-NAT-i-nib Iclusig: /aɪˈkluːsɪɡ/ eye-KLOO-sig |
| Trade names | Iclusig |
| Other names | AP24534 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613029 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| Drug class | Tyrosine-kinase inhibitor[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | >99% (in vitro) |
| Metabolism | Liver (CYP3A4, 2C8, 2D6, 3A5) |
| Elimination half-life | 12–66 hours |
| Excretion | Feces (87%), urine (5%)[2] |
| Identifiers | |
| |
| Chemical and physical data | |
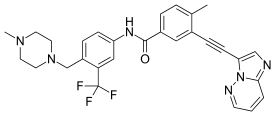
| Formula | C29H27F3N6O |
| Molar mass | 532.571 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ponatinib, sold under the brand name Iclusig, is a medication used to treat chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) which is Philadelphia chromosome–positive (Ph+).[1] It is used when other treatments have failed.[1] It is taken by mouth.[1]
Common side effects include pneumonia, pancreatitis, fever, abdominal pain, heart attack, atrial fibrillation, low platelets, low neutrophils, stroke, kidney problems, and heart failure.[3] Serious blood clots occur in arteries in about 25% of people and veins in about 5% of people.[3] It is a tyrosine-kinase inhibitor which blocks Bcr-Abl.[1][3]
Ponatinib was approved for medical use in the United States in 2012 and Europe in 2013.[1][3] In the United Kingdom it costs the NHS about £5,100 per month.[4] This amount in the United States is about 19,000 USD.[5]
References
[edit]- ^ a b c d e f g h "Ponatinib Monograph for Professionals". Drugs.com. Archived from the original on 6 March 2016. Retrieved 28 October 2021.
- ^ "Iclusig (ponatinib) Tablets, for Oral Use. Full Prescribing Information". ARIAD Pharmaceuticals, Inc. 26 Landsdowne Street, Cambridge, MA 02139-4234. Archived from the original on 17 October 2018. Retrieved 2 October 2016.
- ^ a b c d e "Iclusig". Archived from the original on 13 August 2021. Retrieved 28 October 2021.
- ^ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1041. ISBN 978-0857114105.
- ^ "Iclusig Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 16 April 2021. Retrieved 28 October 2021.
