User:Mr. Ibrahem/Prednisolone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Orapred, PediaPred, Millipred, others |
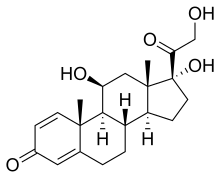
| Other names | 11,17-Dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydrocyclopenta[a] phenanthren-3-one |
| AHFS/Drugs.com | Systemic: Monograph Eyes: Monograph |
| MedlinePlus | a615042 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, topical, eye drop |
| Drug class | Corticosteroid[2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 2–3 hours |
| Excretion | Urine |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C21H28O5 |
| Molar mass | 360.450 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Prednisolone is a steroid medication used to treat certain types of allergies, inflammatory conditions, autoimmune disorders, and cancers.[4][5] Some of these conditions include adrenocortical insufficiency, high blood calcium, rheumatoid arthritis, dermatitis, eye inflammation, asthma, and multiple sclerosis.[5] It is used by mouth, injection into a vein, as a skin cream, and as eye drops.[6][7][5]
Side effects with short-term use include nausea and feeling tired.[4] More severe side effects include psychiatric problems, which may occur in about 5% of people.[8] Common side effects with long term use include bone loss, weakness, yeast infections, and easy bruising.[5] While short-term use in the later part of pregnancy is safe, long-term use or use in early pregnancy is occasionally associated with harm to the baby.[1] It is a glucocorticoid made from hydrocortisone (cortisol).[9]
Prednisolone was discovered and approved for medical use in 1955.[9] It is on the World Health Organization's List of Essential Medicines.[10] It is available as a generic medication.[5] Thewholesale cost in the developing world is about US$0.01 per 5 mg tablet.[11] In 2017, it was the 129th most commonly prescribed medication in the United States, with more than five million prescriptions.[12][13]
References[edit]
- ^ a b c "Prednisolone Use During Pregnancy". Drugs.com. 16 January 2000. Archived from the original on 21 December 2016. Retrieved 9 March 2020.
- ^ a b "Single Drug Information – International Medical Products Price Guide". mshpriceguide. Archived from the original on 29 August 2021. Retrieved 14 August 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 7 August 2020. Retrieved 20 September 2020.
- ^ a b World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 53–54. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c d e "Prednisolone". The American Society of Health-System Pharmacists. Archived from the original on 23 December 2016. Retrieved 8 December 2016.
- ^ "Orapred ODT- prednisolone sodium phosphate tablet, orally disintegrating". DailyMed. 11 September 2019. Archived from the original on 12 May 2021. Retrieved 9 March 2020.
- ^ "Omnipred- prednisolone acetate suspension". DailyMed. 9 September 2019. Archived from the original on 13 May 2021. Retrieved 9 March 2020.
- ^ "Pevanti 10mg Tablets – Summary of Product Characteristics (SPC) – (eMC)". www.medicines.org.uk. 1 December 2014. Archived from the original on 20 December 2016. Retrieved 13 December 2016.
- ^ a b Kim, Kyu-Won; Roh, Jae Kyung; Wee, Hee-Jun; Kim, Chan (2016). Cancer Drug Discovery: Science and History. Springer. p. 169. ISBN 9789402408447. Archived from the original on 2017-09-10.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Prednisolone" (PDF). WHO International Drug Price Indicator Guide, 2014. Archived (PDF) from the original on 10 January 2017. Retrieved 7 December 2017.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ^ "Prednisolone - Drug Usage Statistics". ClinCalc. Archived from the original on 31 October 2021. Retrieved 11 April 2020.
