User:Mr. Ibrahem/Quinine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | US: /ˈkwaɪnaɪn/, /kwɪˈniːn/ or UK: /ˈkwɪniːn/ KWIN-een |
| Trade names | Qualaquin, Quinate, Quinbisul, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682322 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular, intravenous, rectal |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 70–95%[1] |
| Metabolism | Liver (mostly CYP3A4 and CYP2C19-mediated) |
| Elimination half-life | 8–14 hours (adults), 6–12 hours (children)[1] |
| Excretion | Kidney (20%) |
| Identifiers | |
| |
| Chemical and physical data | |
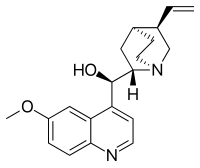
| Formula | C20H24N2O2 |
| Molar mass | 324.424 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 177 °C (351 °F) |
| |
| |
| | |
Quinine is a medication used to treat malaria and babesiosis.[3] This includes the treatment of malaria due to Plasmodium falciparum that is resistant to chloroquine when artesunate is not available.[3][5] While sometimes used for restless legs syndrome, quinine is not recommended for this purpose due to the risk of serious side effects.[3] It can be taken by mouth or intravenously.[3] Malaria resistance to quinine occurs in certain areas of the world.[3] Quinine is also the ingredient in tonic water that gives it its bitter taste.[6]
Common side effects include headache, ringing in the ears, trouble seeing, and sweating.[3] More severe side effects include deafness, low blood platelets, and an irregular heartbeat.[3] Use can make one more prone to sunburn.[3] While it is unclear if use during pregnancy causes harm to the baby, treating malaria during pregnancy with quinine when appropriate is still recommended.[3] Quinine is an alkaloid, a naturally occurring chemical compound.[3] How it works as a medicine is not entirely clear.[3]
Quinine was first isolated in 1820 from the bark of a cinchona tree, which is native to Peru.[3][7][8] Bark extracts had been used to treat malaria since at least 1632 and it was introduced to Spain as early as 1636 by Jesuit missionaries from the New World.[9] Quinine is on the World Health Organization's List of Essential Medicines.[10] The wholesale price in the developing world is about US$1.70 to $3.40 per course of treatment.[11] In the United States a course of treatment costs more than $200.[12]
References[edit]
- ^ a b "Qualaquin (quinine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2 February 2014. Retrieved 29 January 2014.
- ^ "QUININE oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 24 August 2020.
- ^ a b c d e f g h i j k l m "Quinine sulfate". Drugs.com. 2020-02-20. Archived from the original on 2015-12-05. Retrieved 2020-05-14.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 22 September 2020.
- ^ Esu EB, Effa EE, Opie ON, Meremikwu MM (June 2019). "Artemether for severe malaria". The Cochrane Database of Systematic Reviews. 6: CD010678. doi:10.1002/14651858.CD010678.pub3. PMC 6580442. PMID 31210357.
- ^ Olmsted, John; Williams, Gregory M. (1997). Chemistry: The Molecular Science. Jones & Bartlett Learning. p. 137. ISBN 978-0-815-18450-8. Archived from the original on 15 September 2016.
- ^ Willcox, Merlin (28 June 2004). Traditional Medicinal Plants and Malaria. CRC Press. p. 231. ISBN 9780203502327. Archived from the original on 24 February 2020. Retrieved 11 November 2016.
- ^ Cechinel-Filho, Valdir (2012). Plant bioactives and drug discovery : principles, practice, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 2. ISBN 9780470582268. Archived from the original on 4 March 2016.
- ^ Staines, Henry M.; Krishna, Sanjeev (2011). Treatment and Prevention of Malaria : Antimalarial Drug Chemistry, Action and Use. [S.l.]: Springer Verlag. p. 45. ISBN 9783034604796. Archived from the original on 2020-02-22. Retrieved 2017-09-10.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Quinine Sulfate". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 12 January 2016.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 47. ISBN 9781284057560.
