User:Mr. Ibrahem/Tacrolimus
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prograf, Advagraf, Protopic, others |
| Other names | FK-506, fujimycin |
| AHFS/Drugs.com | Systemic: Monograph Topical: Monograph |
| MedlinePlus | a601117 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical, by mouth, intravenous |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 24% (5–67%), less after eating food rich in fat |
| Protein binding | ≥98.8% |
| Metabolism | Liver CYP3A4, CYP3A5 |
| Elimination half-life | 11.3 h post transplant (range 3.5–40.6 h) |
| Excretion | Mostly faecal |
| Identifiers | |
| |
| Chemical and physical data | |
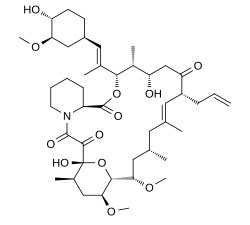
| Formula | C44H69NO12 |
| Molar mass | 804.018 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tacrolimus is a medication used by mouth or by injection to prevent rejection after organ transplant.[2][3] Levels of the medication should be regularly monitored.[3] As a cream it is used for moderate to severe eczema or psoriasis when other treatments are not sufficient.[2][4]
Common side effects when take by mouth or injection include kidney problems, diarrhea, nausea, high blood potassium, and infection.[5] Other side effects may include high blood sugar, neurological problems, lymphoma, and anaphylaxis.[3] Common side effects when used as a cream include itchiness, headache, and red skin.[4] It is unclear if the cream is safe in pregnancy.[6] Tacrolimus is a calcineurin inhibitor.[2] It is believed to works by decreasing the activity of T cells.[3]
Tacrolimus was discovered in 1987, and approved for medical use in the United States in 1994.[5] It is available as a generic medication.[7] In the United Kingdom 50 tablets of 5 mg costs the NHS about 206 pounds in 2020.[2] In the United States this amount costs about 82 USD as of 2020 while 30 grams of 0.1% cream costs about 36 USD.[7][8] It was initially made from a soil sample in Japan that contained the bacterium Streptomyces tsukubaensis.[9]
References[edit]
- ^ a b "Tacrolimus Use During Pregnancy". Drugs.com. 3 October 2019. Retrieved 29 April 2020.
- ^ a b c d BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 868. ISBN 9780857113658.
- ^ a b c d "Tacrolimus Monograph for Professionals". Drugs.com. Retrieved 12 October 2020.
- ^ a b "Tacrolimus topical Monograph for Professionals". Drugs.com. Retrieved 12 October 2020.
- ^ a b "Tacrolimus Monograph for Professionals". Drugs.com. Retrieved 12 October 2020.
- ^ "Tacrolimus topical (Protopic) Use During Pregnancy". Drugs.com. Retrieved 12 October 2020.
- ^ a b "Tacrolimus Prices, Coupons & Savings Tips". GoodRx. Retrieved 12 October 2020.
- ^ "Tacrolimus Prices and Tacrolimus Coupons". GoodRx. Retrieved 12 October 2020.
- ^ Chakravarty, Dilip; Chakravarty, Dilip K.; Lee, W. C. (2010). Liver Transplantation. Boydell & Brewer Ltd. p. 169. ISBN 978-81-8448-770-1.
