User:Mr. Ibrahem/Thiamazole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tapazole, others |
| Other names | thiamazole (INN, BAN); methimazole (USAN); MMI |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682464 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Thioamide[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Protein binding | None |
| Metabolism | Liver |
| Elimination half-life | 5-6 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
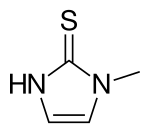
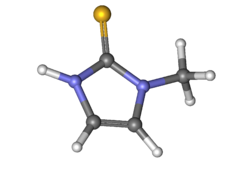
| Formula | C4H6N2S |
| Molar mass | 114.17 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 146 °C (295 °F) |
| Solubility in water | 275[2] mg/mL (20 °C) |
| |
| |
| (verify) | |
Thiamazole, also known as methimazole, is a medication used to treat hyperthyroidism.[1] This includes Graves disease, toxic multinodular goiter, and thyrotoxic crisis.[1] It is taken by mouth.[1] Full effects may take a few weeks to occur.[4]
Common side effects include itchiness, hair loss, nausea, muscle pain, swelling, and abdominal pain.[1] Severe side effects may include low blood cell counts, liver failure, and vasculitis.[1] Use is not recommended during the first trimester of pregnancy but may be used in the second trimester or third trimester.[5] It may be used during breastfeeding.[5] Those who developed significant side effects may also have problems with propylthiouracil.[1] Thiamazole is a thioamide and works by decreasing the production of thyroid hormones.[1]
Thiamazole was approved for medical use in the United States in 1950.[1] It is on the World Health Organization's List of Essential Medicines.[6] It is available as a generic medication.[1] A month supply in the United States has a wholesale cost of about US$6.80.[7] It is also available in Europe and Asia.[8] In 2017, it was the 244th most commonly prescribed medication in the United States, with more than one million prescriptions.[9][10]
References[edit]
- ^ a b c d e f g h i j k l "Methimazole Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 17 July 2019. Retrieved 8 April 2019.
- ^ "DrugBank: Methimazole (DB00763)". drugbank.ca. Archived from the original on 15 January 2017. Retrieved 21 July 2015.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 3 December 2020. Retrieved 8 September 2020.
- ^ Spina, Domenico (2008). The Flesh and Bones of Medical Pharmacology E-Book. Elsevier Health Sciences. p. 74. ISBN 9780723437161. Archived from the original on 29 August 2021. Retrieved 8 April 2019.
- ^ a b "Methimazole Use During Pregnancy". Drugs.com. Archived from the original on 8 April 2019. Retrieved 8 April 2019.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ Jastrzębska, Helena (2015). "Antithyroid drugs". Thyroid Research. 8 (Suppl 1): A12. doi:10.1186/1756-6614-8-S1-A12. PMC 4480840.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ^ "Methimazole - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
