User:Mr. Ibrahem/Vericiguat
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Verquvo |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Soluble guanylate cyclase activator |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
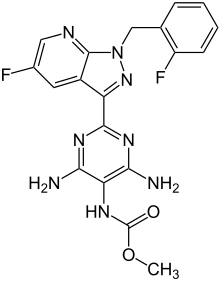
| Formula | C19H16F2N8O2 |
| Molar mass | 426.388 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vericiguat, sold under the brand name Verquvo, is a medication used in heart failure after a recent worsening to reduce the risk of heart related death and hospitalization.[3][5] It is only used in those with an ejection fraction less than 45%.[3] It is taken by mouth.[5]
Common side effects include low blood pressure and low red blood cells.[4][5] Use in pregnancy may harm the baby.[4] It is a soluble guanylate cyclase (sGC) stimulator, and should not be used with other medications in this class.[3][5]
Vericiguat was approved for medical use in the United States and Europe in 2021.[4][5] In the United States it costs about 615 USD per month as of 2022.[6] This amount in the United Kingdom costs the NHS about £100.[7]
References[edit]
- ^ a b "Verquvo". Therapeutic Goods Administration (TGA). 29 November 2021. Archived from the original on 28 December 2021. Retrieved 28 December 2021.
- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Archived from the original on 3 April 2022. Retrieved 13 May 2022.
- ^ a b c d e f "Verquvo- vericiguat tablet, film coated". DailyMed. Archived from the original on 6 September 2021. Retrieved 9 February 2021.
- ^ a b c d e "Drug Trials Snapshot: Verquvo". U.S. Food and Drug Administration (FDA). 8 February 2021. Archived from the original on 9 February 2021. Retrieved 8 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f g h "Verquvo EPAR". European Medicines Agency (EMA). 19 May 2021. Archived from the original on 15 September 2021. Retrieved 14 September 2021.
- ^ "Verquvo". Archived from the original on 23 October 2022. Retrieved 23 October 2022.
- ^ "Vericiguat". SPS - Specialist Pharmacy Service. 17 October 2016. Archived from the original on 3 March 2022. Retrieved 23 October 2022.
