User:Physorg 2013/sandbox
P_org_2013 is sandbox being used for full page
Antiaromaticity is a characteristic of molecules with alternating single and double bonds that have higher π (pi) electron energy than their straight-chain counterparts. Antiaromatic compounds are thus highly unstable and highly reactive. Antiaromatic species do not follow Hückel's rule for aromaticity: having [4n+2] π electrons[1]. Antiaromatic species have [4n] π electrons, but this alone is not diagnostic of antiaromaticity because many species will distort themselves out of a planar geometry to avoid the instability caused by being antiaromatic. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.

Examples of antiaromatic compounds are pentalene (A), biphenylene (B), cyclopentadienyl anion (C). The prototypical example of antiaromaticity, cyclobutadiene, is the subject of debate, with some scientists arguing that antiaromaticity is not a major factor contributing to its destabilization[2]. Cyclooctatetraene appears at first glance to be aromatic, but is an excellent example of a molecule adopting a non-planar geometry to avoid the destabilization that results from antiaromaticity[3]. Because antiaromatic compounds are often short-lived and difficult to work with experimentally, antiaromatic destabilization energy is often modeled by simulation rather than by experimentation. [2]
Examples of Antiaromaticity Affecting Reactivity[edit]
Antiaromatic compounds, often being very unstable, can be highly reactive in order to relieve the antiaromatic destabilization. Cyclobutadiene, for example, rapidly dimerizes with no potential energy barrier via a Diels-Alder reaction to form tricyclooctadiene[4]

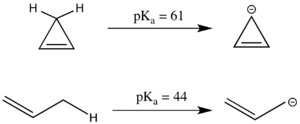
Antiaromaticity can also have a significant effect on pKa. The linear compound 1-propene has a pKa of 44, which is relatively acidic for an sp3 carbon center because the resultant allyl anion can be resonance stabilized. The analogous cyclic system appears to have even more resonance stabilized, as the negative charge can be delocalized across three carbons instead of two. However, the cyclopropenyl anion has 4 π electrons in a cyclic system and in fact has a substantially higher pKa than 1-propene because it is antiaromatic and thus destabilized. [3]. Because antiaromatic compounds are often short-lived and difficult to work with experimentally, antiaromatic destabilization energy is often modeled by simulation rather than by experimentation. [2]
Some antiaromatic compounds are stable, especially larger cyclic systems (in which the antiaromatic destabilization is not as substantial). For example, the aromatic species 1 can be reduced to 2 with a relatively small penalty for forming an antiaromatic system. The antiaromatic 2 does revert to the aromatic species 1 over time by reacting with oxygen in the air because the aromaticity is preferred. [5]

The loss of aromaticity can sometimes be the driving force of a reaction. In the following keto-enol tautomerization, the product enol is more stable than the original ketone even though the ketone contains an aromatic benzene moiety (blue). However, there is also an antiaromatic lactone moiety (green). The relief of antiaromatic destabilization provides a driving force that outweighs even the loss of an aromatic benzene. [4]

Identifying Aromaticity[edit]
Antiaromaticity in NMR Spectra[edit]
The paramagnetic ring current resulting from the electron delocalization in antiaromatic compounds can be observed by NMR. This ring current leads to a deshielding (downfield shift) of nuclei inside the ring and a shielding (upfield shift) of nuclei outside the ring. [12]annulene is an antiaromatic hydrocarbon that is large enough to have protons both inside and outside of the ring. The chemical shift for the protons inside its ring is 5.91 ppm and that for the protons outside the ring is 7.86 ppm, compared to the normal range of 4.5-6.5 ppm for nonaromatic alkenes. This effect is of a smaller magnitude than the corresponding shifts in aromatic compounds. [6]
Many aromatic and antiaromatic compounds (benzene and cyclobutadiene) are too small to have protons inside of the ring, where shielding and deshielding effects can be more diagnostically useful in determining if a compound is aromatic, antiaromatic, or nonaromatic. Nucleus Independent Chemical Shift (NICS) analysis is a method of computing the ring shielding (or deshielding) at the center of a ring system to predict aromaticity or antiaromaticity. A negative NICS value is indicative of aromaticity and a positive value is indicative of antiaromaticity. [7]
References[edit]
- ^ "IUPAC Gold Book: Antiaromaticity". Retrieved 27 October 2013.
- ^ a b c Wu, Judy I-Chia; Mo, Yirong; Evangelista, Francesco Alfredo; von Ragué Schleyer, Paul (June 2012). "Is cyclobutadiene really highly destablilized by antiaromaticity?". Chem. Comm. 48 (67): 8437–8439. doi:10.1039/c2cc33521b. PMID 22801355.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b Anslyn, Eric V. (2006). Modern Physical Organic Chemistry. University Science Books. ISBN 978-1-891389-31-3.
- ^ a b Yi, Li (July 2001). "The Dimerization of Cyclobutadiene. An ab Initio CASSCF Theoretical Study". JACS. 57: 6043–6049. doi:10.1016/S0040-4020(01)00585-3.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) Cite error: The named reference "dimerization" was defined multiple times with different content (see the help page). - ^ A Thiadiazole-Fused N,N-Dihydroquinoxaline: Antiaromatic but Isolable Shaobin Miao, Paul v. R. Schleyer, Judy I. Wu, Kenneth I. Hardcastle, and Uwe H. F. Bunz Org. Lett.; 2007; 9(6) pp 1073 - 1076; (Letter) doi:10.1021/ol070013i
- ^ Alkorta, Ibon (June 1992). "An ab initio study of the NMR properties (absolute shielding and NICS) of a series of significant aromatic and antiaromatic compouds". Teterahedron. 118: 880–885. doi:10.1021/ja921663m.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ What is aromaticity? Paul von Ragué Schleyer and Haijun Jiao Pure & Appl. Chem., Vol. 68, No. 2, pp. 209-218, 1996 Link

