User:Szeichner/sandbox
 | This is a user sandbox of Szeichner. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |

| |
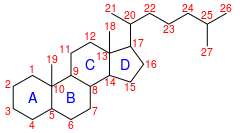 IUPAC numbering[1]
| |
| Names | |
|---|---|
| IUPAC name
(8R,9S,10S,13R,14S,17R)-10,13-Dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C27H48 | |
| Molar mass | 372.681 g·mol−1 |
| Density | 0.911 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cholestane is a saturated tetracyclic triterpene. This carbon-27 biomarker is produced by diagenesis of cholesterol and is one of the most abundant biomarkers in the rock record[2]. Presence of cholestane in environmental samples are commonly interpreted as an indicator of animal life and/or traces of O2, as animals are known for exclusively producing cholesterol, and thus has been used to draw evolutionary relationships between ancient organisms of unknown phylogenetic origin and modern metazoan taxa[3]. Cholestane is made in low abundance by other organisms (e.g., rhodophytes). It is often found in analysis of organic compounds in petroleum.
Background[edit]
Cholestane is a saturated C-27 animal biomarker often found in petroleum deposits. It is a diagenetic product of cholesterol, which is an organic molecule made primarily by animals and make up ~30% of animal cell membranes. Cholesterol is responsible for membrane rigidity and fluidity, as well as intracellular transport, cell signaling and nerve conduction. In humans, it is also the precursor for hormones (i.e., estrogen, testosterone). It is synthesized via squalene and naturally assumes a specific stereochemical orientation (3β-ol, 5α (H), 14α (H), 17α (H), 20R). Maintaining stereochemistry of natural cholesterol is typical through diagenetic processes, but cholestane can be found in the fossil record with many stereochemical configurations.
Cholestane in the fossil record is often interpreted as an indicator of ancient animal life and are often used by geochemists and geobiologists to reconstruct animal evolution (particularly in very early Earth history; i.e., Ediacaran[3], neo-Proterozoic and Proterozoic [4],[5]). Oxygen is required to produce cholesterol; thus, the presence of cholestane suggests some trace of oxygen in the paleoenvironment. However, cholestane is not exclusively derived from diagenesis of animal-derived biomolecules; cholestane has also been associated with the presence of rhodophytes[6].
Preservation[edit]
Cholesterol has 256 stereoisomers, but only one of them is formed naturally in production of cholesterol (3β-ol, 5α (H), 14α (H), 17α (H), 20R) and is therefore the primary stereoisomer of interest for cholestane measurements. Deviations from this stereochemistry often reflects diagenesis, thermal maturation and preservation bias.


Diagenesis typically leads to the loss of functional groups and double bonds in organic molecules. For cholestane specifically, diagenesis of cholesterol to cholestane produces a molecule that is fully saturated compared to its steroid counterpart. This process occurs without the loss or gain of carbon atoms and therefore can serve as an indicator of the original steroid produced by the organism in the environment[8].
Additional diagenetic processes can further alter the cholestane molecule. For instance, cholestane is susceptible to stereochemical shifts over time from its natural isomer. These changes can be the effect of thermal or microbial alteration. Thermal alteration can cause changes in stereochemistry at both the C20 chiral center, as well as the hydrogen atoms. The ratio of R/S stereoisomers is typically reported as a measure of “thermal maturity”[9]. In contrast, conversion of 5α → β configuration reflects anaerobic microbial activity[3], and can be understood through isotope labeling experiments on controlled microbe experiments metabolizing the steroid of interest[10][11]. A previous study demonstrated that there are two reactions that can produce loss of the cholesterol double bond—(1) direct reduction of double bond or (2) production of ketone prior to reduction of double bond—resulting in distinct C5 isomers[10]. The 14 and 17α hydrogen sites are more stable and undergo changes to β configuration in much lower abundances than the 5 hydrogen site.
Thermal alteration can also cause loss of the alkane side chain through β-fission that cleaves the carbon-carbon double bond[12]. A previous experiment demonstrated that over 4 weeks at 300°C, cholestane underwent 17% decomposition of its alkane side chain. In contrast, the polycyclic structure is very thermally stable. Diagenetic processes can also cause methyl shifts and aromatization.
Measurement Techniques[edit]
GC/MS[edit]
Cholestane can be extracted from samples and measured on the GC/MS to quantify relative abundance to other organic compounds. This measurement is done by extraction of the steranes into a non-polar solvent (e.g., dichloromethane or chloroform) and purified into a “saturates” fraction using silica gas chromatography. Cholestane isomers will elute from the column based on molecular weight and various stereochemistry, which makes traditional mass spectrometry challenging due to close co-elution of isomers. Alternatively, one can measure cholestane using GC/MS/MS experiments which target the m/z fragment 217 (from molecular ion 372) and improves identification of specific isomers.
δ13C isotope ratios[edit]
δ13C values of cholestane reflect the carbon isotope composition of the animals that created the original cholesterol molecules. Animal carbon isotope composition is typically understood to be a function of their diet[13]; therefore, carbon isotope composition of cholestane would reflect this original diet value as well. More generally, steranes can be used as an indicator of environmental shifts. A previous study has presented δ13C values of steranes versus hopanes and used it to propose changes in the photic zone over the course of the Miocene, as changes in the isotope value must be either a result of dissolved inorganic carbon within the water or biological isotope fractionation[9].
Case studies[edit]
Early life biomarkers[edit]
Presence of cholestane not only indicates presence of animals, but is often used in conjunction with other biomarkers to note the rise of distinct taxa in the fossil record. A previous study measured relative abundance in cholestane versus other triterpenoid biomarkers to demonstrate the rise of algae during the Neoproterozoic[4].
Tracing the actual origins of cholestane within the fossil record is challenging, as most of the rocks from that time period are heavily metamorphosed and thus potential biomarkers are thermally altered. A previous study tried to constrain the source of cholestane to a specific Ediacaran fossil (Dickinsonia) to provide constraints to the taxonomic classification of Ediacaran biota as evolutionary preludes to metazoan life[3].
See also[edit]
References[edit]
- ^ The Nomenclature of Steroids, IUPAC
- ^ Peters, Kenneth E. (Kenneth Eric), 1950- Walters, C. C. Moldowan, J. M. (J. Michael), 1946- (2005). The biomarker guide. Cambridge University Press. ISBN 9781107089440. OCLC 668201944.
{{cite book}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ a b c d Bobrovskiy, Ilya; Hope, Janet M.; Ivantsov, Andrey; Nettersheim, Benjamin J.; Hallmann, Christian; Brocks, Jochen J. (2018-09-20). "Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals". Science. 361 (6408): 1246–1249. doi:10.1126/science.aat7228. ISSN 0036-8075.
- ^ a b Brocks, Jochen J.; Jarrett, Amber J. M.; Sirantoine, Eva; Hallmann, Christian; Hoshino, Yosuke; Liyanage, Tharika (2017-08). "The rise of algae in Cryogenian oceans and the emergence of animals". Nature. 548 (7669): 578–581. doi:10.1038/nature23457. ISSN 0028-0836.
{{cite journal}}: Check date values in:|date=(help) - ^ Summons, Roger E; Brassell, Simon C; Eglinton, Geoffrey; Evans, Evan; Horodyski, Robert J; Robinson, Neil; Ward, David M (1988-11). "Distinctive hydrocarbon biomarkers from fossiliferous sediment of the Late Proterozoic Walcott Member, Chuar Group, Grand Canyon, Arizona". Geochimica et Cosmochimica Acta. 52 (11): 2625–2637. doi:10.1016/0016-7037(88)90031-2. ISSN 0016-7037.
{{cite journal}}: Check date values in:|date=(help) - ^ Summons, Roger E.; Erwin, Douglas H. (2018-09-20). "Chemical clues to the earliest animal fossils". Science. 361 (6408): 1198–1199. doi:10.1126/science.aau9710. ISSN 0036-8075.
- ^ Bobrovskiy, Ilya; Hope, Janet M.; Ivantsov, Andrey; Nettersheim, Benjamin J.; Hallmann, Christian; Brocks, Jochen J. (2018-09-20). "Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals". Science. 361 (6408): 1246–1249. doi:10.1126/science.aat7228. ISSN 0036-8075.
- ^ Grantham, P.J.; Wakefield, L.L. (1988-01). "Variations in the sterane carbon number distributions of marine source rock derived crude oils through geological time". Organic Geochemistry. 12 (1): 61–73. doi:10.1016/0146-6380(88)90115-5. ISSN 0146-6380.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Schoell, M.; Schouten, S.; Damste, J. S. S.; de Leeuw, J. W.; Summons, R. E. (1994-02-25). "A Molecular Organic Carbon Isotope Record of Miocene Climate Changes". Science. 263 (5150): 1122–1125. doi:10.1126/science.263.5150.1122. ISSN 0036-8075.
- ^ a b Mermoud, F.; Wünsche, L.; Clerc, O.; Gülaçar, F.O.; Buchs, A. (1984-01). "Steroidal ketones in the early diagenetic transformations of Δ5 sterols in different types of sediments". Organic Geochemistry. 6: 25–29. doi:10.1016/0146-6380(84)90023-8. ISSN 0146-6380.
{{cite journal}}: Check date values in:|date=(help) - ^ Taylor, Craig D.; Smith, Steven O.; Gagosian, Robert B. (1981-11). "Use of microbial enrichments for the study of the anaerobic degradation of cholesterol". Geochimica et Cosmochimica Acta. 45 (11): 2161–2168. doi:10.1016/0016-7037(81)90068-5. ISSN 0016-7037.
{{cite journal}}: Check date values in:|date=(help) - ^ Mango, Frank D. (1990-01). "The origin of light cycloalkanes in petroleum". Geochimica et Cosmochimica Acta. 54 (1): 23–27. doi:10.1016/0016-7037(90)90191-m. ISSN 0016-7037.
{{cite journal}}: Check date values in:|date=(help) - ^ Hayes, John M. (2001-12-31), "3. Fractionation of Carbon and Hydrogen Isotopes in Biosynthetic Processes", Stable Isotope Geochemistry, De Gruyter, pp. 225–278, ISBN 9781501508745, retrieved 2019-05-20
External links[edit]
- Cholestanes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
