Usnic acid
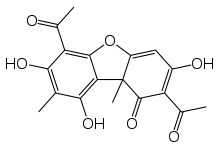
| |
| Names | |
|---|---|
| IUPAC names
2,6-Diacetyl-7,9-dihydroxy-8,9b-dimethyl-1,3(2H,9bh)
-dibenzofurandione | |
| Other names
usneine, usninic acid, usniacin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.310 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H16O7 | |
| Molar mass | 344.315 g/mol |
| Melting point | 204 °C (399 °F; 477 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Usnic acid is a naturally occurring dibenzofuran derivative found in several lichen species with the formula C18H16O7. It was first isolated by German scientist W. Knop in 1844[2] and first synthesized between 1933-1937 by Curd and Robertson.[3] Usnic acid was identified in many genera of lichens including Usnea, Cladonia, Hypotrachyna, Lecanora, Ramalina, Evernia, Parmelia and Alectoria. Although it is generally believed that usnic acid is exclusively restricted to lichens, in a few unconfirmed isolated cases the compound was found in kombucha tea and non-lichenized ascomycetes.[4][5]
At normal conditions, usnic acid is a bitter, yellow, solid substance.[1] It is known to occur in nature in both the d- and l-forms as well as a racemic mixture. Salts of usnic acid are called usnates (e.g. copper usnate).
Biological role in lichens

Usnic acid is a secondary metabolite in lichens whose role has not been completely elucidated. It is believed that usnic acid protects the lichen from adverse effects of sunlight exposure and deters grazing animals with its bitter taste.
Uses and properties
Lichen extracts containing usnic acid have been utilized in medicine, perfumery, cosmetics, and ecology.
Usnic acid possesses a wide range of interesting biological properties. It is a potent antibiotic effective against Gram-positive bacteria, including Staphylococcus, Streptococcus, and Pneumococcus, other bacteria such as Mycobacterium tuberculosis, and some pathogenic fungi. It also exhibits antiviral, antiprotozoal, antimitotic, anti-inflammatory and analgesic activity. Other characteristics, like ultraviolet absorption, preserving properties, antigrowth, antiherbivore and anti-insect properties, have also been demonstrated.
Usnic acid has been included as an ingredient in creams, powders, toothpastes, mouthwash, deodorants, hair shampoos and sunscreen products. In some of these preparations, usnic acid is employed as an active principle, in others as a preservative.
Usnic acid and its salt form, sodium usniate, have been marketed in the US as an ingredient in food supplements for use in weight reduction, although unsupported by solid scientific proof. These supplements, if taken according to label instructions, can supply daily oral doses of 10–1350 mg for adults.
Safety
Usnic acid and its salts are idiosyncratically associated with severe hepatotoxicity and liver failure.[6] Daily oral intake of 300–1350 mg over a period of weeks has led to severe hepatotoxicity in a number of persons.[7][8]
Sodium usniate was one ingredient in a product called "Lipokinetix", that was claimed to induce weight loss via an increase in metabolic rate. Lipokinetix has been the topic of an FDA warning in the USA,[9] due to potential hepatotoxicity, although it is unclear yet if any toxicity would be attributable to the aforementioned salt. Lipokinetix also contained norephedrine (PPA), caffeine, yohimbine and 3,5-Diiodothyronine.
Pharmacology
Usnic acid has been found to have adrenergic activity in both frog and earthworm nerve junction models in preliminary research.[10]
Analytics
It is possible to determine the content of usnic acid in lichen extract using reversed-polarity capillary zone electrophoretic analysis.[11]
References
- ^ a b Michael Ash; Irene Ash (2004). Handbook of preservatives. Synapse Info Resources. p. 5856. ISBN 978-1-890595-66-1. Retrieved 5 August 2010.
- ^ Knop, W (1844). "Chemisch-physiologische Untersuchung uber die Flechten". Justus Lieb. Ann. Chern. 49: 103–124. doi:10.1002/jlac.18440490202.
- ^ Robertson, A.; Curd, F. H. (1933). "277. Usnic acid. Part III. Usnetol, usnetic acid, and pyrousnic acid". J. Chem. Soc.: 1173. doi:10.1039/jr9330001173.
- ^ Cocchietto, Moreno; Skert, Nicola; Nimis, Pier; Sava, Gianni (2002). "A review on usnic acid, an interesting natural compound". Naturwissenschaften. 89 (4): 137–146. doi:10.1007/s00114-002-0305-3. ISSN 0028-1042.
- ^ Blanc, Philippe J. (1996). "Characterization of the tea fungus metabolites". Biotechnology Letters. 18 (2): 139–142. doi:10.1007/BF00128667. ISSN 0141-5492.
- ^ Yellapu RK, Mittal V, Grewal P, Fiel M, Schiano T (2011). "Acute liver failure caused by 'fat burners' and dietary supplements: a case report and literature review". Canadian Journal of Gastroenterology. 25 (3): 157–60. PMC 3076034. PMID 21499580.
{{cite journal}}:|access-date=requires|url=(help) - ^ Hsu, LM; Huang, YS; Chang, FY; Lee, SD (Jul 2005). "'Fat burner' herb, usnic acid, induced acute hepatitis in a family". J Gastroenterol Hepatol. 20 (7): 1138–9. doi:10.1111/j.1440-1746.2005.03855.x.
- ^ Sanchez W, Maple JT, Burgart LJ, Kamath PS. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin Proc. 2006 Apr;81(4):541-4.
- ^ "Safety Alerts for Human Medical Products > Lipokinetix". MedWatch: The FDA Safety Information and Adverse Event Reporting Program. U.S. Food and Drug Administration. November 20, 2001. Retrieved 5 December 2012.
FDA has received multiple reports of persons who developed liver injury or liver failure while using Lipokinetix. The product contains norephedrine (also known as phenylpropanolamine or PPA), caffeine, yohimbine, diiodothyronine, and sodium usniate.
- ^ Harris N. J. (1961), Honors Thesis, Clark University, Worcester, Massachusetts
- ^ Kreft, S. et Štrukelj, B. Reversed-polarity capillary zone electrophoretic analysis of usnic acid. Electrophoresis, 22:2755-2757 http://pubs.acs.org/doi/abs/10.1021/ac990198%2B
External links
- http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=search&db=pcsubstance&term=usnic%20acid
- http://bioweb.ucr.edu/ChemMine/view.php?TYPE=1&i_id=996789[dead link]
- http://web.archive.org/web/20110608210146/http://ntp.niehs.nih.gov/index.cfm?objectid=E87D09E6-BDB5-82F8-FF11A36F2EEA025F
- http://query.nytimes.com/gst/fullpage.html?res=9802E2DD163FF937A35750C0A9659C8B63
