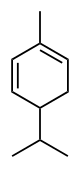
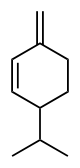
Phellandrene
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
(α): 2-Methyl-5-(propan-2-yl)cyclohexa-1,3-diene
(β): 3-Methylidene-6-(propan-2-yl)cyclohex-1-ene | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.014.121 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties[1] | |||
| C10H16 | |||
| Molar mass | 136.24 g/mol | ||
| Appearance | Colorless oil (α and β) | ||
| Density | α: 0.846 g/cm3 β: 0.85 g/cm3 | ||
| Boiling point | α: 171-172 °C β: 171-172 °C | ||
| Insoluble (α and β) | |||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H226, H304 | |||
| P210, P233, P240, P241, P242, P243, P280, P301+P310, P303+P361+P353, P331, P370+P378, P403+P235, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Phellandrenes are a pair of organic compounds that have a similar molecular structure and similar chemical properties. α-Phellandrene and β-phellandrene are cyclic monoterpenes and are double-bond isomers. In α-phellandrene, both double bonds are endocyclic and in β-phellandrene, one of them is exocyclic. Both are insoluble in water, but miscible with diethyl ether.
α-Phellandrene was named after Eucalyptus phellandra, now called Eucalyptus radiata, from which it can be isolated.[2] It is also a constituent of the essential oil of Eucalyptus dives.[3] β-Phellandrene has been isolated from the oil of water fennel and Canada balsam oil.
The phellandrenes are used in fragrances because of their pleasing aromas. The odor of β-phellandrene has been described as peppery-minty and slightly citrusy.
The α-phellandrene isomer can form hazardous and explosive peroxides on contact with air at elevated temperatures.[4]
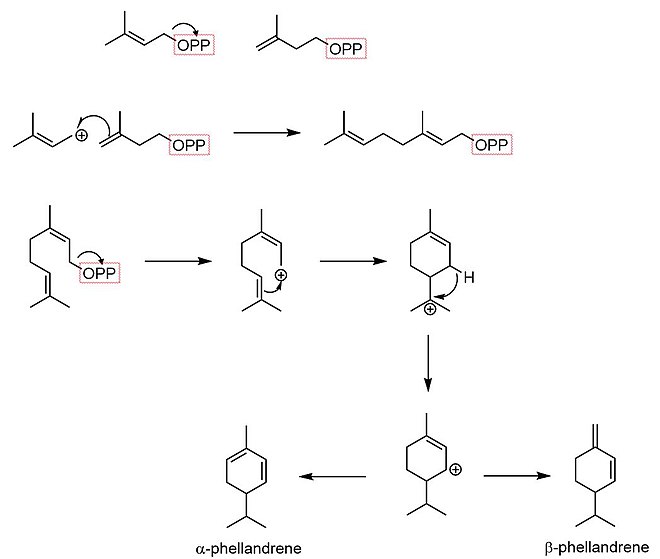
Biosynthesis
[edit]The biosynthesis of phellandrene begins with dimethylallyl pyrophosphate and isopentenyl pyrophosphate condensing in an SN1 reaction to form geranyl pyrophosphate. The resultant monoterpene undergoes cyclization to form a menthyl cationic species. A hydride shift then forms an allylic carbocation. Finally, an elimination reaction occurs at one of two positions, yielding either α-phellandrene or β-phellandrene.[5]
References
[edit]- ^ The Merck Index, 12th Edition, 7340, 7341
- ^ Jacobs, S.W.L., Pickard, J., Plants of New South Wales, 1981, ISBN 0-7240-1978-2.
- ^ Boland, D. J., Brophy, J. J., and A. P. N. House, Eucalyptus Leaf Oils, 1991, ISBN 0-909605-69-6.
- ^ Urben, Peter (2007). Bretherick's Handobook of Reactive Chemical Hazards. Vol. 1 (7 ed.). Butterworth-Heinemann. p. 1154.
- ^ Dewick, Paul M. (9 March 2009). Medicinal natural products : a biosynthetic approach (3rd ed.). Chichester, West Sussex, United Kingdom. ISBN 9780470741689. OCLC 259265604.
{{cite book}}: CS1 maint: location missing publisher (link)