Ferrous

In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ferrous or the prefix ferro- is often used to specify such compounds, as in ferrous chloride for iron(II) chloride (FeCl2). The adjective ferric is used instead for iron(III) salts, containing the cation Fe3+. The word ferrous is derived from the Latin word ferrum, meaning "iron".
In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) abbreviated as Fe2+, although more precise descriptions include other ligands such as water and halides. Iron(II) centres occur in coordination complexes, such as in the anion ferrocyanide, [Fe(CN)6]4−, where six cyanide ligands are bound the metal centre; or, in organometallic compounds, such as the ferrocene [Fe(C2H5)2], where two cyclopentadienyl anions are bound to the FeII centre.
Ferrous ions in biology
[edit]All known forms of life require iron.[1] Many proteins in living beings contain iron(III) centers. Examples of such metalloproteins include hemoglobin, ferredoxin, and the cytochromes. In many of these proteins, Fe(II) converts reversibly to Fe(III).[2]
Insufficient iron in the human diet causes anemia. Animals and humans can obtain the necessary iron from foods that contain it in assimilable form, such as meat. Other organisms must obtain their iron from the environment. However, iron tends to form highly insoluble iron(III) oxides/hydroxides in aerobic (oxygenated) environment, especially in calcareous soils. Bacteria and grasses can thrive in such environments by secreting compounds called siderophores that form soluble complexes with iron(III), that can be reabsorbed into the cell. (The other plants instead encourage the growth around their roots of certain bacteria that reduce iron(III) to the more soluble iron(II).)[3]
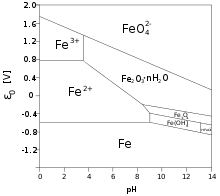
In contrast to iron(III) aquo complexes, iron(II) aquo complexes are soluble in water near neutral pH.[citation needed] Ferrous iron is, however, oxidized by the oxygen in air, converting to iron(III).[4]
Ferrous salts and complexes
[edit]Typically iron(II) salts, like the "chloride" are aquo complexes with the formulas [Fe(H2O)6]2+, as found in ferrous ammonium sulfate.[5]
The aquo ligands on iron(II) complexes are labile. It reacts with 1,10-phenanthroline to give the blue iron(II) derivative:
When metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(II) chloride is formed, with release of hydrogen gas, by the reaction
- Fe0 + 2 H+ → Fe2+ + H2
Iron(II) is oxidized by hydrogen peroxide to iron(III), forming a hydroxyl radical and a hydroxide ion in the process. This is the Fenton reaction. Iron(III) is then reduced back to iron(II) by another molecule of hydrogen peroxide, forming a hydroperoxyl radical and a proton. The net effect is a disproportionation of hydrogen peroxide to create two different oxygen-radical species, with water (H+ + OH−) as a byproduct.[6]
| Fe2+ + H2O2 → Fe3+ + HO• + OH− | (1) |
| Fe3+ + H2O2 → Fe2+ + HOO• + H+ | (2) |
The free radicals generated by this process engage in secondary reactions, which can degrade many organic and biochemical compounds.

Ferrous minerals and other solids
[edit]
Iron(II) is found in many minerals and solids. Examples include the sulfide and oxide, FeS and FeO. These formulas are deceptively simple because these sulfides and oxides are often nonstoichiometric. For example, "ferrous sulfide" can refer to the 1:1 species (mineral name troilite) or a host of Fe-deficient derivatives (pyrrhotite). The mineral magnetite ("lode stone") is a mixed-valence compound with both Fe(II) and Fe(III), Fe3O4.
Bonding
[edit]
Iron(II) is a d6 center, meaning that the metal has six "valence" electrons in the 3d orbital shell. The number and type of ligands bound to iron(II) determine how these electrons arrange themselves. With the so-called "strong field ligands" such as cyanide, the six electrons pair up. Thus ferrocyanide ([Fe(CN)6]4− has no unpaired electrons, meaning it is a low-spin complex. With so-called "weak field ligands" such as water, four of the six electrons are unpaired, meaning it is a high-spin complex. Thus aquo complex [Fe(H2O)6]2+ is paramagnetic. With chloride, iron(II) forms tetrahedral complexes, e.g. [FeCl4]2−. Tetrahedral complexes are high-spin complexes.
Gallery
[edit]-
Ferrous nitrate hexahydrate, Fe(NO3)2·6H2O
-
Ferrous oxalate dihydrate, Humboldtine, FeC2O4·2H2O
-
Vivianite, Ferrous phosphate octahydrate, Fe3(PO4)2·8H2O
-
Ferrous sulfate heptahydrate, Melanterite, FeSO4·7H2O
-
Ferrous sulfide, Troilite, FeS
-
Ferrous silicate, Ferrosilite, FeSiO3
See also
[edit]- Ferric – The element iron in its +3 oxidation state — [Iron(III)] compounds
- Ferromagnetism – Mechanism by which materials form into and are attracted to magnets
- Ferrous metal recycling – Recyclable materials left over from manufactured products after their use
- Iron(II) oxide – Inorganic compound with the formula FeO (ferrous oxide)
- Iron(II) bromide – Chemical compound (ferrous bromide)
- Steelmaking – Process for producing steel
References
[edit]- ^ "Iron integral to the development of life on Earth – and the possibility of life on other planets". University of Oxford. 7 December 2021. Retrieved 9 May 2022.
- ^ Berg, Jeremy Mark; Lippard, Stephen J. (1994). Principles of bioinorganic chemistry. Sausalito, Calif: University Science Books. ISBN 0-935702-73-3.
- ^ H. Marschner and V. Römheld (1994): "Strategies of plants for acquisition of iron". Plant and Soil, volume 165, issue 2, pages 261–274. doi:10.1007/BF00008069
- ^ Petsch, S.T. (2014). "10.11 - The Global Oxygen Cycle". In Holland, H.D.; Turekian, K.K. (eds.). Treatise on Geochemistry. Reference Module in Earth Systems and Environmental Sciences. Vol. 10 (Second ed.). Elsevier. pp. 437–473. doi:10.1016/B978-0-08-095975-7.00811-1. ISBN 978-0-08-095975-7.
- ^ Earnshaw, A.; Greenwood, N. N. (1997). Chemistry of the elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Tang, Zhongmin; Zhao, Peiran; Wang, Han; Liu, Yanyan; Bu, Wenbo (2021). "Biomedicine Meets Fenton Chemistry". Chemical Reviews. 121 (4): 1981–2019. doi:10.1021/acs.chemrev.0c00977. PMID 33492935. S2CID 231712587.






