Melanotan II
| |
| Names | |
|---|---|
| Pronunciation | /mɛˈlænoʊtæn/ ⓘ |
| Systematic IUPAC name
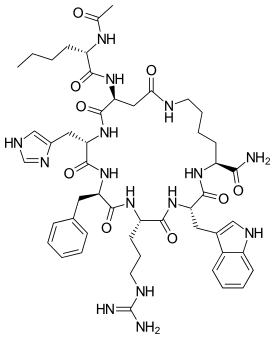
L-Lysinamide, N-acetyl-L-norleucyl-L-alpha-aspartyl-L-histidyl-D-phenylalanyl-L-arginyl-L-tryptophyl-, cyclic (2-7)-peptide | |
| Other names
List of other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | melanotan-II |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C50H69N15O9 | |
| Molar mass | 1024.180 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Melanotan II is a synthetic analogue of the peptide hormone α-melanocyte-stimulating hormone (α-MSH) that stimulates melanogenesis and increases sexual arousal.
It was under development as drug candidate for female sexual dysfunction and erectile dysfunction, but clinical development ceased by 2003, and as of 2018, no product containing melanotan II was marketed and all commercial development had ceased.[1]
Unlicensed and untested Melanotan II is found on the internet and marketed for tanning, though side effects such as uneven pigmentation (it makes already uneven pigmentation more noticeable), new nevi (moles), and darkening or enlargement of existing moles have been reported and have led to medical authorities discouraging its use. There has been no scientific study into the long term and permanent side effects the use of this peptide may cause.[2][3]
Synthesis
[edit]In the synthesis of melanotan II, an ε-amino group of lysine and an γ-carboxy group of aspartic acid have their orthogonal protection removed before undergoing a carbodiimide mediated lactamization, leading to an intermediate. This intermediate, when attached to N-acetylnorleucine, forms melanotan II. The entire process can be accomplished in 12 steps with an overall yield of 2.6%, and the product is more than 90% pure without preparative chromatography.[4]
Mechanism of action
[edit]Melanotan II acts as a non-selective agonist of the melanocortin receptors MC1, MC3, MC4, and MC5.[5]
Melanotan II produces melanogenesis by activation of the MC1 receptor, whereas its clinically documented sexual effects are thought to be related to its ability to activate the MC4 receptor (though the MC3 is thought to also possibly be involved).[6][7]
Other effects of melanotan II, mostly regarded as adverse effects, include flushing, nausea, vomiting, stretching, yawning, and loss of appetite (the last via activation of MC4).[8][9]
History
[edit]Research in the early 1960s showed that in rats, administration of α-MSH caused sexual arousal, and work on this continued in many labs up through the 1980s, when scientists at the University of Arizona began attempting to develop α-MSH and analogs as potential sunless tanning agents, and synthesized and tested several analogs, including melanotan-I and melanotan II.[7][10]
Early in the research process one of the scientists, who was conducting experiments on himself with an early tool compound, melanotan II, injected himself with twice the dose he intended to and got an eight-hour erection, along with nausea and vomiting.[7]
As a tanning agent, melanotan I (now known as afamelanotide) was licensed by Competitive Technologies, a technology transfer company operating on behalf of the University of Arizona, to an Australian startup called Epitan,[11][12] which changed its name to Clinuvel in 2006.[13]
As a sexual dysfunction agent, melanotan II was licensed by Competitive Technologies to Palatin Technologies.[10] Palatin ceased development of melanotan II in 2000 and synthesized, patented, and began to develop bremelanotide, a likely metabolite of melanotan II that differs in that it has a carboxy group where melanotan II has an amide.[7][14] Competitive Technologies sued Palatin for breach of contract and tried to claim ownership of bremelanotide;[14] the parties settled in 2008 with Palatin retaining rights to bremelanotide, returning rights to melanotan II to Competitive Technologies, and paying US$800,000.[15]
Society and culture
[edit]Numerous products are sold online and in gyms and beauty salons as "melanotan" or "melanotan-1" or "melanotan-2" in their marketing.[16][17][18]
The unregulated products are not legal to be sold for human usage in any jurisdiction.[19][20][21][22]
Starting in 2007, health agencies in various countries began issuing warnings against their use.[23][24][25][26][27][28]
See also
[edit]References
[edit]- ^ "Melanotan II". AdisInsight. Retrieved 13 Jan 2018.
- ^ Brennan R, Wells JS, Van Hout MC (September 2017). "The injecting use of image and performance-enhancing drugs (IPED) in the general population: a systematic review". Health & Social Care in the Community. 25 (5): 1459–1531. doi:10.1111/hsc.12326. PMID 26806443. S2CID 20159129.
- ^ Habbema L, Halk AB, Neumann M, Bergman W (October 2017). "Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review". International Journal of Dermatology. 56 (10): 975–980. doi:10.1111/ijd.13585. PMID 28266027. S2CID 37255702.
- ^ Ryakhovsky, Vladimir V; Khachiyan, Georgy A; Kosovova, Nina F; Isamiddinova, Elena F; Ivanov, Andrey S (30 October 2008). "The first preparative solution phase synthesis of melanotan II". Beilstein Journal of Organic Chemistry. 4: 39. doi:10.3762/bjoc.4.39. PMC 2587946. PMID 19043625.
- ^ Wikberg JE (2001). "Melanocortin receptors: new opportunities in drug discovery". Expert Opinion on Therapeutic Patents. 11 (1): 61–76. doi:10.1517/13543776.11.1.61. ISSN 1354-3776. S2CID 86254068.
- ^ Norris DO, Lopez KH (25 November 2010). Hormones and Reproduction of Vertebrates. Academic Press. pp. 4–. ISBN 978-0-08-095809-5.
- ^ a b c d King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H (2007). "Melanocortin receptors, melanotropic peptides and penile erection". Curr Top Med Chem. 7 (11): 1098–1106. doi:10.2174/1568026610707011111. PMC 2694735. PMID 17584130.
- ^ Plant TM, Zeleznik AJ (15 November 2014). Knobil and Neill's Physiology of Reproduction: Two-Volume Set. Academic Press. pp. 2230–. ISBN 978-0-12-397769-4.
- ^ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (28 September 2011). Campbell-Walsh Urology. Elsevier Health Sciences. pp. 743–. ISBN 978-1-4557-2298-3.
- ^ a b Hadley ME (October 2005). "Discovery that a melanocortin regulates sexual functions in male and female humans". Peptides. 26 (10): 1687–9. doi:10.1016/j.peptides.2005.01.023. PMID 15996790. S2CID 22559801.
- ^ "EpiTan focuses on Melanotan, a potential blockbuster". The Pharma Letter. 1 November 2004.
- ^ Hadley ME, Dorr RT (April 2006). "Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization". Peptides. 27 (4): 921–30. doi:10.1016/j.peptides.2005.01.029. PMID 16412534. S2CID 21025287.
- ^ "Epitan changes name to Clinuvel, announces new clinical program". LabOnline. 27 February 2006.
- ^ a b "Press Release: Palatin Technologies Refutes Competitive Technologies Contention of Material Breach". Palatin Technologies via PR Newswire. September 12, 2007.
- ^ "Press Release: Palatin Technologies Announces Litigation Settlement With Competitive Technologies". Palatin Technologies via PR Newswire. January 22, 2008.
- ^ "Believe It Or Not 'Tanorexia' A Very Real Problem". WCBS-TV, CBS. 2009-05-20. Archived from the original on May 21, 2009. Retrieved 2009-07-23.
- ^ "Fools Gold". Cosmopolitan (Australia). 2009-06-14. Archived from the original on 2009-09-12. Retrieved 2009-07-25.
- ^ Madrigal A (2009-01-29). "Suntan Drug Greenlighted for Trials". Wired. Archived from the original on 5 May 2009. Retrieved 2009-04-11.
- ^ "Tanning drug a health risk". Herald Sun. 2009-10-31. Archived from the original on 2010-12-29. Retrieved 2009-10-31.
- ^ Langan EA, Nie Z, Rhodes LE (September 2010). "Melanotropic peptides: more than just 'Barbie drugs' and 'sun-tan jabs'?". The British Journal of Dermatology. 163 (3): 451–5. doi:10.1111/j.1365-2133.2010.09891.x. PMID 20545686. S2CID 8203334.
- ^ Langan EA, Ramlogan D, Jamieson LA, Rhodes LE (January 2009). "Change in moles linked to use of unlicensed "sun tan jab"". BMJ. 338: b277. doi:10.1136/bmj.b277. PMID 19174439. S2CID 27838904.
- ^ "Risky tan jab warnings 'ignored'". BBC. 2009-02-18. Archived from the original on 21 February 2009. Retrieved 2009-03-04.
- ^ "Warning against the product Melanotan". Danish Medicines Agency. 2008. Retrieved 2008-08-11.
- ^ ""Tan jab" is an unlicensed medicine and may not be safe". MHRA. 2008. Archived from the original on 2008-12-18. Retrieved 2008-11-17.
- ^ "US Lab Research Inc Warning letter". U.S. Food and Drug Administration. 2009-01-29. Archived from the original on 10 July 2009. Retrieved 2009-07-23.
- ^ "Melanotan Powder for Injection". Notice Information: – Warning – 27 February 2009. Irish Medicines Board. 2009. Retrieved 2009-02-02.
- ^ "Legemiddelverket advarer mot bruk av Melanotan". Norwegian Medicines Agency. 2007-12-13. Archived from the original on 17 April 2009. Retrieved 2009-03-11.
- ^ "Melanotan – farlig og ulovlig brunfarge". Norwegian Medicines Agency. 2009-01-23. Archived from the original on 17 April 2009. Retrieved 2009-03-11.
