Helicene: Difference between revisions
split off circulene |
add Chem Rev, add earlier contributions |
||
| Line 1: | Line 1: | ||
[[File:Hexahelicene-from-xtal-3D-vdW.png|right|150px|Hexahelicene]] |
[[File:Hexahelicene-from-xtal-3D-vdW.png|right|150px|Hexahelicene]] |
||
'''Helicenes''' in [[organic chemistry]] are [[aromatic ortho substituent|ortho-condensed]] [[Polycyclic compound|polycyclic]] [[Aromaticity|aromatic compounds]] in which [[Benzene|benzene rings]] or other aromatics are angularly [[annulation|annulated]] to give [[helix|helically]]-shaped [[molecules]]. The chemistry of helicenes has attracted continuing attention because of their unique structural, [[Spectroscopy|spectral]], and [[Optics|optical]] features.<ref>''Diels-Alder Additions of Benzynes within Helicene Skeletons'' David Zhigang Wang, Thomas J. Katz, James Golen, and Arnold L. Rheingold J. Org. Chem.; '''2004'''; 69(22) pp 7769 - 7771 |
'''Helicenes''' in [[organic chemistry]] are [[aromatic ortho substituent|ortho-condensed]] [[Polycyclic compound|polycyclic]] [[Aromaticity|aromatic compounds]] in which [[Benzene|benzene rings]] or other aromatics are angularly [[annulation|annulated]] to give [[helix|helically]]-shaped [[molecules]]. The chemistry of helicenes has attracted continuing attention because of their unique structural, [[Spectroscopy|spectral]], and [[Optics|optical]] features.<ref>''Helicenes: Synthesis and Applications'' Yun Shen and Chuan-Feng Chen Chemical Reviews Article ASAP {{DOI|10.1021/cr200087r}}</ref> <ref>''Diels-Alder Additions of Benzynes within Helicene Skeletons'' David Zhigang Wang, Thomas J. Katz, James Golen, and Arnold L. Rheingold J. Org. Chem.; '''2004'''; 69(22) pp 7769 - 7771 {{doi|10.1021/jo048707h}}</ref> |
||
Helicenes are notable for having [[Chirality (chemistry)|chirality]] while lacking both [[tetrahedral molecular geometry|asymmetric carbons]] and [[chiral center]]s. Helicenes' chirality results from the fact that clockwise and counterclockwise helices are non-[[superimposition|superimposable]] – this is an example of [[axial chirality]]. |
Helicenes are notable for having [[Chirality (chemistry)|chirality]] while lacking both [[tetrahedral molecular geometry|asymmetric carbons]] and [[chiral center]]s. Helicenes' chirality results from the fact that clockwise and counterclockwise helices are non-[[superimposition|superimposable]] – this is an example of [[axial chirality]]. |
||
==Background== |
==Background== |
||
[[Image:-6-helicene.svg|right|100px|[6]helicene]] |
|||
| ⚫ | |||
The first helicene structure was reported by [[Jakob Meisenheimer]] in 1903 as the reduction product of [[2-nitronaphtalene]] <ref>Meisenheimer, J. and Witte, K. (1903), ''Reduction von 2-Nitronaphtalin''. Berichte der deutschen chemischen Gesellschaft, 36: 4153–4164. {{doi|10.1002/cber.19030360481}}</ref>. [5]helicene was synthesised in 1918 by Weitzenböck & Klingler. <ref>''Synthese der isomeren Kohlenwasserstoffe 1, 2–5, 6-Dibenzanthracen und 3, 4–5, 6-Dibenzphenanthren'' Richard Weitzenböck and Albert Klingler Monatshefte für Chemie / Chemical Monthly Volume 39, Number 5, 315-323, {{DOI|10.1007/BF01524529}} </ref> The first [6]helicene (also called ''hexahelicene'') was [[Chemical synthesis|synthesized]] by [[Melvin Spencer Newman|M. S. Newman]] and D. Lednicer in 1955 via a scheme that closed the two central rings by [[Friedel-Crafts reaction|Friedel-Crafts cyclization]] of [[carboxylic acid]] compounds. <ref> A NEW REAGENT FOR RESOLUTION BY COMPLEX FORMATION; THE RESOLUTION OF PHENANTHRO-[3,4-c]PHENANTHRENE Melvin S. Newman, Wilson B. Lutz, and Daniel Lednicer Journal of the American Chemical Society 1955 77 (12), 3420-3421 {{DOI|10.1021/ja01617a097}}</ref> <ref> ''The Synthesis and Resolution of Hexahelicene'' Melvin S. Newman and Daniel Lednicer Journal of the American Chemical Society 1956 78 (18), 4765-4770 |
|||
| ⚫ | {{DOI|10.1021/ja01599a060}}</ref> Since then, several methods for synthesizing helicenes have been reported. Today, the synthesis of helicenes with different lengths and [[substituent]]s is possible. The oxidative [[Electrocyclic reaction|photocyclization]] of a [[stilbene]]-type [[Precursor (chemistry)|precursor]] is used most often as the key step. The longest helicene, [14]helicene, was prepared in 1975 by this method. |
||
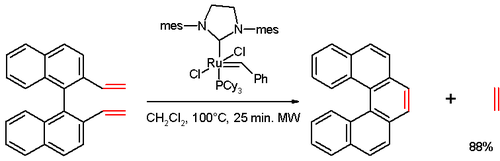
In one study,<ref>''Preparation of Helicenes through Olefin Metathesis '' Shawn K. Collins, Alain Grandbois, Martin P. Vachon, Julie Côté [[Angewandte Chemie International Edition]] Volume 45, Issue 18 , Pages 2923 - 2926 '''2006''' |
In one study,<ref>''Preparation of Helicenes through Olefin Metathesis '' Shawn K. Collins, Alain Grandbois, Martin P. Vachon, Julie Côté [[Angewandte Chemie International Edition]] Volume 45, Issue 18 , Pages 2923 - 2926 '''2006''' {{doi|10.1002/anie.200504150}}</ref> [5]helicene was synthesized in an [[olefin metathesis]] reaction of a divinyl compound (prepared from [[1,1'-bi-2-naphthol]] (BINOL) in several steps), with [[Grubbs' catalyst|Grubbs' second generation catalyst]]: |
||
[[Image:Helicene olefin Metathesis.png|center|500px|Helicene synthesis by olefin metathesis]] |
[[Image:Helicene olefin Metathesis.png|center|500px|Helicene synthesis by olefin metathesis]] |
||
Revision as of 18:36, 29 December 2011

Helicenes in organic chemistry are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped molecules. The chemistry of helicenes has attracted continuing attention because of their unique structural, spectral, and optical features.[1] [2]
Helicenes are notable for having chirality while lacking both asymmetric carbons and chiral centers. Helicenes' chirality results from the fact that clockwise and counterclockwise helices are non-superimposable – this is an example of axial chirality.
Background
![[6]helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/e/ed/-6-helicene.svg/100px--6-helicene.svg.png)
The first helicene structure was reported by Jakob Meisenheimer in 1903 as the reduction product of 2-nitronaphtalene [3]. [5]helicene was synthesised in 1918 by Weitzenböck & Klingler. [4] The first [6]helicene (also called hexahelicene) was synthesized by M. S. Newman and D. Lednicer in 1955 via a scheme that closed the two central rings by Friedel-Crafts cyclization of carboxylic acid compounds. [5] [6] Since then, several methods for synthesizing helicenes have been reported. Today, the synthesis of helicenes with different lengths and substituents is possible. The oxidative photocyclization of a stilbene-type precursor is used most often as the key step. The longest helicene, [14]helicene, was prepared in 1975 by this method.
In one study,[7] [5]helicene was synthesized in an olefin metathesis reaction of a divinyl compound (prepared from 1,1'-bi-2-naphthol (BINOL) in several steps), with Grubbs' second generation catalyst:
Polyacenes are the linear 1,3-fused or meta-analogues of helicenes. Conceptually related compounds are the circulenes.
External links
References
- ^ Helicenes: Synthesis and Applications Yun Shen and Chuan-Feng Chen Chemical Reviews Article ASAP doi:10.1021/cr200087r
- ^ Diels-Alder Additions of Benzynes within Helicene Skeletons David Zhigang Wang, Thomas J. Katz, James Golen, and Arnold L. Rheingold J. Org. Chem.; 2004; 69(22) pp 7769 - 7771 doi:10.1021/jo048707h
- ^ Meisenheimer, J. and Witte, K. (1903), Reduction von 2-Nitronaphtalin. Berichte der deutschen chemischen Gesellschaft, 36: 4153–4164. doi:10.1002/cber.19030360481
- ^ Synthese der isomeren Kohlenwasserstoffe 1, 2–5, 6-Dibenzanthracen und 3, 4–5, 6-Dibenzphenanthren Richard Weitzenböck and Albert Klingler Monatshefte für Chemie / Chemical Monthly Volume 39, Number 5, 315-323, doi:10.1007/BF01524529
- ^ A NEW REAGENT FOR RESOLUTION BY COMPLEX FORMATION; THE RESOLUTION OF PHENANTHRO-[3,4-c]PHENANTHRENE Melvin S. Newman, Wilson B. Lutz, and Daniel Lednicer Journal of the American Chemical Society 1955 77 (12), 3420-3421 doi:10.1021/ja01617a097
- ^ The Synthesis and Resolution of Hexahelicene Melvin S. Newman and Daniel Lednicer Journal of the American Chemical Society 1956 78 (18), 4765-4770 doi:10.1021/ja01599a060
- ^ Preparation of Helicenes through Olefin Metathesis Shawn K. Collins, Alain Grandbois, Martin P. Vachon, Julie Côté Angewandte Chemie International Edition Volume 45, Issue 18 , Pages 2923 - 2926 2006 doi:10.1002/anie.200504150
