Sinapinic acid: Difference between revisions
m clean up PMC, replaced: {{PMCID|PMC → {{PMCID| using AWB (8060) |
use cite templates |
||
| Line 40: | Line 40: | ||
Sinapic acid can form dimers with itself (one structure) and [[ferulic acid]] (three different structures) in cereal cell walls and therefore may have a similar influence on cell-wall structure to that of the [[diferulic acids]].<ref name="Bunzel 2003">{{cite journal |author=Bunzel M, Ralph J, Kim H, Lu F, Ralph SA, Marita JM, Hatfield RD, Steinhart H |title=Sinapate dehydrodimers and sinapate-ferulate heterodimers in cereal dietary fibre |journal=J. Agric. Food Chem. |volume=51 |pages=1427–1434 |year=2003 |doi=10.1021/jf020910v |pmid=12590493 |issue=5}}</ref> |
Sinapic acid can form dimers with itself (one structure) and [[ferulic acid]] (three different structures) in cereal cell walls and therefore may have a similar influence on cell-wall structure to that of the [[diferulic acids]].<ref name="Bunzel 2003">{{cite journal |author=Bunzel M, Ralph J, Kim H, Lu F, Ralph SA, Marita JM, Hatfield RD, Steinhart H |title=Sinapate dehydrodimers and sinapate-ferulate heterodimers in cereal dietary fibre |journal=J. Agric. Food Chem. |volume=51 |pages=1427–1434 |year=2003 |doi=10.1021/jf020910v |pmid=12590493 |issue=5}}</ref> |
||
[[Sinapine]] is an alkaloidal amine found in black mustard seeds. It is considered a [[choline]] ester of [[sinapic acid]].<ref> |
[[Sinapine]] is an alkaloidal amine found in black mustard seeds. It is considered a [[choline]] ester of [[sinapic acid]].<ref>{{cite pmid|16655775}}</ref> |
||
== Natural occurrences == |
== Natural occurrences == |
||
Sinapinic acid can be found in [[vinegar]].<ref> |
Sinapinic acid can be found in [[vinegar]].<ref>{{cite doi|10.1007/BF01192948}}</ref> |
||
== See also == |
== See also == |
||
Revision as of 22:50, 15 April 2012
| |
| Names | |
|---|---|
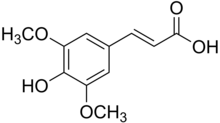
| IUPAC name
3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid
| |
| Other names
Sinapinic acid
Sinapic acid 3,5-Dimethoxy-4-hydroxycinnamic acid 4-Hydroxy-3,5-dimethoxycinnamic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H12O5 | |
| Molar mass | 224.21 g/mol |
| Melting point | 203–205 °C (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sinapinic acid, or sinapic acid (Sinapine - Origin: L. Sinapi, sinapis, mustard, Gr., cf. F. Sinapine.), is a small naturally occurring hydroxycinnamic acid. It is a member of the phenylpropanoid family. It is a commonly used matrix in MALDI mass spectrometry.[1][2] It is a useful matrix for a wide variety of peptides and proteins. It serves well as a matrix for MALDI due to its ability to absorb laser radiation and to also donate protons (H+) to the analyte of interest.
Sinapic acid can form dimers with itself (one structure) and ferulic acid (three different structures) in cereal cell walls and therefore may have a similar influence on cell-wall structure to that of the diferulic acids.[3]
Sinapine is an alkaloidal amine found in black mustard seeds. It is considered a choline ester of sinapic acid.[4]
Natural occurrences
Sinapinic acid can be found in vinegar.[5]
See also
References
- ^ Beavis RC, Chait BT (1989). "Matrix-assisted laser-desorption mass spectrometry using 355 nm radiation". Rapid Commun. Mass Spectrom. 3 (12): 436–9. doi:10.1002/rcm.1290031208. PMID 2520224.
- ^ Beavis RC, Chait BT (1989). "Cinnamic acid derivatives as matrices for ultraviolet laser desorption mass spectrometry of proteins". Rapid Commun. Mass Spectrom. 3 (12): 432–5. doi:10.1002/rcm.1290031207. PMID 2520223.
- ^ Bunzel M, Ralph J, Kim H, Lu F, Ralph SA, Marita JM, Hatfield RD, Steinhart H (2003). "Sinapate dehydrodimers and sinapate-ferulate heterodimers in cereal dietary fibre". J. Agric. Food Chem. 51 (5): 1427–1434. doi:10.1021/jf020910v. PMID 12590493.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16655775, please use {{cite journal}} with
|pmid=16655775instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/BF01192948, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/BF01192948instead.
