Tunneling nanotube: Difference between revisions
consistent citation formatting |
Role in Mitochondrial Transfer section Tags: use of predatory open access journal Visual edit |
||
| Line 20: | Line 20: | ||
One study found that cell-to-cell contact was necessary for the formation of nanotube bridges between [[T cell|T cells]].<ref name="sowinski01" /> [[p53]] activation has also been implicated as a necessary mechanism for the development of TNTs, as the downstream genes up-regulated by p53 (namely [[EGFR]], [[Akt]], [[PI3K]], and [[mTOR]]) were found to be involved in nanotube formation via hydrogen peroxide treatment and serum starvation.<ref>{{cite journal | vauthors = Wang Y, Cui J, Sun X, Zhang Y | title = Tunneling-nanotube development in astrocytes depends on p53 activation | journal = Cell Death and Differentiation | volume = 18 | issue = 4 | pages = 732–42 | date = April 2011 | pmid = 21113142 | pmc = 3131904 | doi = 10.1038/cdd.2010.147 }}</ref> [[Connexin-43]] has shown to promote connection between [[bone marrow stromal cells]] (BMSCs) and [[Alveolus|alveolar]] epithelial cells, leading to the formation of nanotubes.<ref>{{cite journal | vauthors = Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J | display-authors = 6 | title = Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury | journal = Nature Medicine | volume = 18 | issue = 5 | pages = 759–65 | date = April 2012 | pmid = 22504485 | pmc = 3727429 | doi = 10.1038/nm.2736 }}</ref> |
One study found that cell-to-cell contact was necessary for the formation of nanotube bridges between [[T cell|T cells]].<ref name="sowinski01" /> [[p53]] activation has also been implicated as a necessary mechanism for the development of TNTs, as the downstream genes up-regulated by p53 (namely [[EGFR]], [[Akt]], [[PI3K]], and [[mTOR]]) were found to be involved in nanotube formation via hydrogen peroxide treatment and serum starvation.<ref>{{cite journal | vauthors = Wang Y, Cui J, Sun X, Zhang Y | title = Tunneling-nanotube development in astrocytes depends on p53 activation | journal = Cell Death and Differentiation | volume = 18 | issue = 4 | pages = 732–42 | date = April 2011 | pmid = 21113142 | pmc = 3131904 | doi = 10.1038/cdd.2010.147 }}</ref> [[Connexin-43]] has shown to promote connection between [[bone marrow stromal cells]] (BMSCs) and [[Alveolus|alveolar]] epithelial cells, leading to the formation of nanotubes.<ref>{{cite journal | vauthors = Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J | display-authors = 6 | title = Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury | journal = Nature Medicine | volume = 18 | issue = 5 | pages = 759–65 | date = April 2012 | pmid = 22504485 | pmc = 3727429 | doi = 10.1038/nm.2736 }}</ref> |
||
Cellular stress induced by [[rotenone]] or [[Tumor necrosis factor alpha|TNF-α]] was also shown to induce TNT formation between epithelial cells.<ref>{{cite journal | vauthors = Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A | display-authors = 6 | title = Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy | journal = The EMBO Journal | volume = 33 | issue = 9 | pages = 994–1010 | date = May 2014 | pmid = 24431222 | pmc = 4193933 | doi = 10.1002/embj.201386030 }}</ref> Inflammation by [[Lipopolysaccharide|lipopolysaccharides]] or [[Interferon gamma|interferon-γ]] has shown to increase the expression of proteins related to TNT formation.<ref name=":3">{{cite journal | vauthors = Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, Ito M, Watarai H, Hazelett CC, Yeaman C, Ohno H | display-authors = 6 | title = M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex | journal = Nature Cell Biology | volume = 11 | issue = 12 | pages = 1427–32 | date = December 2009 | pmid = 19935652 | doi = 10.1038/ncb1990 }}</ref> |
Cellular stress induced by [[rotenone]] or [[Tumor necrosis factor alpha|TNF-α]] was also shown to induce TNT formation between epithelial cells.<ref name=":4">{{cite journal | vauthors = Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A | display-authors = 6 | title = Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy | journal = The EMBO Journal | volume = 33 | issue = 9 | pages = 994–1010 | date = May 2014 | pmid = 24431222 | pmc = 4193933 | doi = 10.1002/embj.201386030 }}</ref> Inflammation by [[Lipopolysaccharide|lipopolysaccharides]] or [[Interferon gamma|interferon-γ]] has shown to increase the expression of proteins related to TNT formation.<ref name=":3">{{cite journal | vauthors = Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, Ito M, Watarai H, Hazelett CC, Yeaman C, Ohno H | display-authors = 6 | title = M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex | journal = Nature Cell Biology | volume = 11 | issue = 12 | pages = 1427–32 | date = December 2009 | pmid = 19935652 | doi = 10.1038/ncb1990 }}</ref> |
||
=== Nanotube Inhibition === |
=== Nanotube Inhibition === |
||
| Line 26: | Line 26: | ||
[[Tumor necrosis factor superfamily|TNFAIP2]], also called M-Sec, is known to mediate TNT formation, and knockdown of this protein by [[Short hairpin RNA|shRNA]] reduced TNT formation in [[Epithelium|epithelial cells]] by about two-thirds.<ref name=":3" /> |
[[Tumor necrosis factor superfamily|TNFAIP2]], also called M-Sec, is known to mediate TNT formation, and knockdown of this protein by [[Short hairpin RNA|shRNA]] reduced TNT formation in [[Epithelium|epithelial cells]] by about two-thirds.<ref name=":3" /> |
||
== Role in Mitochondrial Transfer == |
|||
Tunneling nanotubes have been implicated as one mechanism by which whole [[Mitochondrion|mitochondria]] can be [[Horizontal transfer of mitochondria|transferred]] from cell to cell.<ref name=":0" /> Mitochondrial damage appears to be the main trigger for the formation of TNTs in order to traffic entire mitochondria,<ref>{{Cite journal|last=Torralba|first=Daniel|last2=Baixauli|first2=Francesc|last3=Sánchez-Madrid|first3=Francisco|date=2016|title=Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer|url=https://www.frontiersin.org/articles/10.3389/fcell.2016.00107/full|journal=Frontiers in Cell and Developmental Biology|language=English|volume=4|doi=10.3389/fcell.2016.00107|issn=2296-634X|pmc=PMC5039171|pmid=27734015}}</ref> though the exact threshold of damage necessary to induce TNT formation is yet unknown. The maximum speed of mitochondria traveling over TNTs was found to be about 80 nm/s, lower than the measured speed of 100-1400 nm/s of axonal transport of mitochondria; this could be due to the smaller diameter of TNTs inhibiting mitochondrial migration.<ref>{{Cite journal|last=Wang|first=X.|last2=Gerdes|first2=H.-H.|date=2015-07|title=Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells|url=https://www.nature.com/articles/cdd2014211|journal=Cell Death & Differentiation|language=en|volume=22|issue=7|pages=1181–1191|doi=10.1038/cdd.2014.211|issn=1476-5403|pmc=PMC4572865|pmid=25571977}}</ref> |
|||
In one study, Ahmad ''et al''. used four lines of mesenchymal stem cells, each expressing either a differing phenotype of the [[Rho family of GTPases|Rho-GTPase]] Miro1; a higher level of Miro1 was associated with more efficient [[Horizontal transfer of mitochondria|mitochondrial transfer]] via TNTs.<ref name=":4" /> Several studies have shown, through the selective blockage of TNT formation, that TNTs are a primary mechanism for the trafficking of whole mitochondria between heterogeneous cells.<ref>{{Cite journal|last=Pasquier|first=Jennifer|last2=Guerrouahen|first2=Bella S.|last3=Al Thawadi|first3=Hamda|last4=Ghiabi|first4=Pegah|last5=Maleki|first5=Mahtab|last6=Abu-Kaoud|first6=Nadine|last7=Jacob|first7=Arthur|last8=Mirshahi|first8=Massoud|last9=Galas|first9=Ludovic|last10=Rafii|first10=Shahin|last11=Le Foll|first11=Frank|date=2013-04-10|title=Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance|url=https://doi.org/10.1186/1479-5876-11-94|journal=Journal of Translational Medicine|volume=11|issue=1|pages=94|doi=10.1186/1479-5876-11-94|issn=1479-5876|pmc=PMC3668949|pmid=23574623}}</ref><ref>{{Cite journal|last=Lu|first=Jinjin|last2=Zheng|first2=Xiufen|last3=Li|first3=Fan|last4=Yu|first4=Yang|last5=Chen|first5=Zhong|last6=Liu|first6=Zheng|last7=Wang|first7=Zhihua|last8=Xu|first8=Hua|last9=Yang|first9=Weimin|date=2017-01-17|title=Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells|url=http://www.oncotarget.com/index.php?journal=oncotarget&page=article&op=view&path[]=14695&path[]=46902|journal=Oncotarget|volume=8|issue=9|pages=15539–15552|doi=10.18632/oncotarget.14695|issn=1949-2553|pmc=PMC5362504|pmid=28107184}}</ref><ref>{{Cite journal|last=Li|first=Xiang|last2=Zhang|first2=Yuelin|last3=Yeung|first3=Sze C.|last4=Liang|first4=Yingmin|last5=Liang|first5=Xiaoting|last6=Ding|first6=Yue|last7=Ip|first7=Mary S. M.|last8=Tse|first8=Hung-Fat|last9=Mak|first9=Judith C. W.|last10=Lian|first10=Qizhou|date=2014-04-16|title=Mitochondrial Transfer of Induced Pluripotent Stem Cell–Derived Mesenchymal Stem Cells to Airway Epithelial Cells Attenuates Cigarette Smoke–Induced Damage|url=https://www.atsjournals.org/doi/full/10.1165/rcmb.2013-0529OC|journal=American Journal of Respiratory Cell and Molecular Biology|volume=51|issue=3|pages=455–465|doi=10.1165/rcmb.2013-0529OC|issn=1044-1549}}</ref> |
|||
== Similar Structures == |
== Similar Structures == |
||
Revision as of 14:28, 29 November 2019
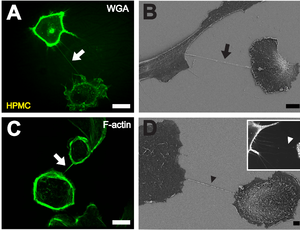
B Depiction of a TNT (black arrow) between two cells with scanning electron microscopy one hour after cell plating. Scale bar: 10 μm.
C F-actin staining by fluorescently labeled phalloidin showing actin being present in TNTs between individual HPMCs (white arrow). Scale bar: 20 μm.
D Scanning electron microscope picture of a substrate-associated filopodia-like extension as potential TNT precursor (black arrowhead). The insert shows a fluorescence microscopic image of substrate associated filopodia-like protrusions approaching a neighboring cell (white arrowhead). Scale bar: 2 μm.[1]
A tunneling nanotube (TNT) or membrane nanotube is a term that has been applied to protrusions that extend from the plasma membrane which enable different animal cells to touch over long distances, sometimes over 100 μm between T cells.[2][3][4] Two types of structures have been called nanotubes. The first type are less than 0.7 micrometers in diameter, contain actin and carry portions of plasma membrane between cells in both directions. The second type are larger (>0.7 μm), contain both actin and microtubules and can carry components of the cytoplasm between cells, such as vesicles and organelles.[5] The diameter of TNTs ranges from 50 to 200 μm and they can reach lengths of several cell diameters.[6] These structures may be involved in cell-to-cell communication,[7] transfer of nucleic acids between cells in a tissue,[8] and the spread of pathogens or toxins such as HIV[3] and prions.[9] TNTs have observed lifetimes ranging from a few minutes up to several hours.[10]
Some dendritic cells and THP-1 monocytes have been shown to connect via tunneling nanotubes and display evidence of calcium flux when exposed to bacterial or mechanical stimuli. TNT-mediated signaling has shown to produce spreading in target cells, similar to the lamellipodia produced when dendritic cells are exposed to bacterial products. The TNTs demonstrated in this study propagated at initial speed of 35 micrometers/second and have shown to connect THP-1 monocytes with nanotubules up to 100 micrometers long.[11]
Vesicular transport in membrane nanotubes has been modeled utilizing a continuum approach.[12] A variety of synthetic nanotubes, based on stacking of cyclic peptides and other cyclic molecules, have been investigated.[13]
History
Membrane nanotubes were first described in a 1999 Cell article examining the development of Drosophila melanogaster wing imaginal discs.[14] More recently, a Science article published in 2004 described structures that connected various types of immune cell together, as well as connections between cells in tissue culture.[6][15] Since these publications, more TNT-like structures have been recorded, containing varying levels of F-actin, microtubules, and other components, but remaining relatively homogenous in terms of composition.[10]
Nanotube Formation
Several mechanisms may be involved in nanotube formation. These include molecular controls as well as cell-to-cell interactions.
Two primary mechanisms for TNT formation have been proposed. The first involves cytoplasmic protrusions extending from one cell to another, where they fuse with the membrane of the target cell.[6] The other is that, as two previously connected cells move away from one another, TNTs remain as bridges between the two cells.[3][16]
Nanotube Induction
The formation of cytonemes towards a BnL-FGF gradient has been observed, suggesting that chemotactic controls may induce the formation of TNT-like structures.[14] A supporting finding is that phosphatidylserine exposure guided TNT formation from mesenchymal stem cells (MSCs) to a population of injured cells.[17] The protein S100A4 and its receptor have been shown to guide the direction of TNT growth, as p53 activates caspase 3 to cleave S100A4 in the initiating cell, thereby generating a gradient in which the target cell has higher amounts of the protein.[18]
One study found that cell-to-cell contact was necessary for the formation of nanotube bridges between T cells.[3] p53 activation has also been implicated as a necessary mechanism for the development of TNTs, as the downstream genes up-regulated by p53 (namely EGFR, Akt, PI3K, and mTOR) were found to be involved in nanotube formation via hydrogen peroxide treatment and serum starvation.[19] Connexin-43 has shown to promote connection between bone marrow stromal cells (BMSCs) and alveolar epithelial cells, leading to the formation of nanotubes.[20]
Cellular stress induced by rotenone or TNF-α was also shown to induce TNT formation between epithelial cells.[21] Inflammation by lipopolysaccharides or interferon-γ has shown to increase the expression of proteins related to TNT formation.[22]
Nanotube Inhibition
TNT-like structures called streamers did not form when cultured with cytochalasin D, an F-actin depolymerizing compound,[23] and a separate study using cytochalasin B found impacted TNT formation without the destruction of existing TNTs.[24] Latrunculin-B, another F-actin depolymerizing compound, was found to completely block TNT formation.[6] Blocking CD38, which had been implicated in the release of mitochondria by astrocytes,[25] also significantly decreased TNT formation.[26]
TNFAIP2, also called M-Sec, is known to mediate TNT formation, and knockdown of this protein by shRNA reduced TNT formation in epithelial cells by about two-thirds.[22]
Role in Mitochondrial Transfer
Tunneling nanotubes have been implicated as one mechanism by which whole mitochondria can be transferred from cell to cell.[6] Mitochondrial damage appears to be the main trigger for the formation of TNTs in order to traffic entire mitochondria,[27] though the exact threshold of damage necessary to induce TNT formation is yet unknown. The maximum speed of mitochondria traveling over TNTs was found to be about 80 nm/s, lower than the measured speed of 100-1400 nm/s of axonal transport of mitochondria; this could be due to the smaller diameter of TNTs inhibiting mitochondrial migration.[28]
In one study, Ahmad et al. used four lines of mesenchymal stem cells, each expressing either a differing phenotype of the Rho-GTPase Miro1; a higher level of Miro1 was associated with more efficient mitochondrial transfer via TNTs.[21] Several studies have shown, through the selective blockage of TNT formation, that TNTs are a primary mechanism for the trafficking of whole mitochondria between heterogeneous cells.[29][30][31]
Similar Structures
A structure called a cytoneme enables exchanges between signaling centers. Cytonemes, however, do not always connect two cells and can act simply as environmental sensors.[23]
Plasmodesmata have been identified as functional channels interconnecting plant cells,[32] and stromules interconnect plastids.[33]
Myopodia are actin-rich cytoplasmic extensions which have been observed in embryonic Drosophila. Similar structures have been observed in Xenopus and mouse models.[10] Actin-containing cellular protrusions dubbed "streamers" have been observed in cultured B cells.[23]
References
- ^ Ranzinger J, Rustom A, Abel M, Leyh J, Kihm L, Witkowski M, et al. (2011-12-27). Bereswill S (ed.). "Nanotube action between human mesothelial cells reveals novel aspects of inflammatory responses". PLOS ONE. 6 (12): e29537. Bibcode:2011PLoSO...629537R. doi:10.1371/journal.pone.0029537. PMC 3246504. PMID 22216308.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Abounit S, Zurzolo C (March 2012). "Wiring through tunneling nanotubes--from electrical signals to organelle transfer" (PDF). Journal of Cell Science. 125 (Pt 5): 1089–98. doi:10.1242/jcs.083279. PMID 22399801.
- ^ a b c d Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, et al. (February 2008). "Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission". Nature Cell Biology. 10 (2): 211–9. doi:10.1038/ncb1682. PMID 18193035.
- ^ Davis DM, Sowinski S (June 2008). "Membrane nanotubes: dynamic long-distance connections between animal cells". Nature Reviews. Molecular Cell Biology. 9 (6): 431–6. doi:10.1038/nrm2399. PMID 18431401.
- ^ Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. (December 2006). "Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria". Journal of Immunology. 177 (12): 8476–83. doi:10.4049/jimmunol.177.12.8476. PMID 17142745.
- ^ a b c d e Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (February 2004). "Nanotubular highways for intercellular organelle transport". Science. 303 (5660): 1007–10. Bibcode:2004Sci...303.1007R. doi:10.1126/science.1093133. PMID 14963329.
- ^ Onfelt B, Davis DM (November 2004). "Can membrane nanotubes facilitate communication between immune cells?". Biochemical Society Transactions. 32 (Pt 5): 676–8. doi:10.1042/BST0320676. PMID 15493985.
- ^ Belting M, Wittrup A (December 2008). "Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease". The Journal of Cell Biology. 183 (7): 1187–91. doi:10.1083/jcb.200810038. PMC 2606965. PMID 19103810.
- ^ Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. (March 2009). "Prions hijack tunnelling nanotubes for intercellular spread". Nature Cell Biology. 11 (3): 328–36. doi:10.1038/ncb1841. PMID 19198598.
- ^ a b c Gurke S, Barroso JF, Gerdes HH (May 2008). "The art of cellular communication: tunneling nanotubes bridge the divide". Histochemistry and Cell Biology. 129 (5): 539–50. doi:10.1007/s00418-008-0412-0. PMC 2323029. PMID 18386044.
- ^ Watkins SC, Salter RD (September 2005). "Functional connectivity between immune cells mediated by tunneling nanotubules". Immunity. 23 (3): 309–18. doi:10.1016/j.immuni.2005.08.009. PMID 16169503.
- ^ Kuznetsov AV (August 2011). "Modeling bidirectional transport of quantum dot nanoparticles in membrane nanotubes". Mathematical Biosciences. 232 (2): 101–9. doi:10.1016/j.mbs.2011.04.008. PMID 21609723.
- ^ Rodríguez-Vázquez, Nuria; Fuertes, Alberto; Amorín, Manuel; Granja, Juan R. (2016). "Chapter 14. Bioinspired Artificial Sodium and Potassium Ion Channels". In Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel (eds.). The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. Vol. 16. Springer. pp. 485–556. doi:10.1007/978-4-319-21756-7_14 (inactive 2019-11-24).
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: DOI inactive as of November 2019 (link) - ^ a b Ramírez-Weber FA, Kornberg TB (May 1999). "Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs". Cell. 97 (5): 599–607. doi:10.1016/S0092-8674(00)80771-0. PMID 10367889.
- ^ Onfelt B, Nedvetzki S, Yanagi K, Davis DM (August 2004). "Cutting edge: Membrane nanotubes connect immune cells". Journal of Immunology. 173 (3): 1511–3. doi:10.4049/jimmunol.173.3.1511. PMID 15265877.
- ^ Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W (March 2007). "Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission". Nature Cell Biology. 9 (3): 310–5. doi:10.1038/ncb1544. PMC 2628976. PMID 17293854.
- ^ Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C (March 2014). "Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer". Microvascular Research. 92: 10–8. doi:10.1016/j.mvr.2014.01.008. PMID 24486322.
- ^ Sun X, Wang Y, Zhang J, Tu J, Wang XJ, Su XD, et al. (December 2012). "Tunneling-nanotube direction determination in neurons and astrocytes". Cell Death & Disease. 3 (12): e438. doi:10.1038/cddis.2012.177. PMC 3542613. PMID 23222508.
- ^ Wang Y, Cui J, Sun X, Zhang Y (April 2011). "Tunneling-nanotube development in astrocytes depends on p53 activation". Cell Death and Differentiation. 18 (4): 732–42. doi:10.1038/cdd.2010.147. PMC 3131904. PMID 21113142.
- ^ Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. (April 2012). "Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury". Nature Medicine. 18 (5): 759–65. doi:10.1038/nm.2736. PMC 3727429. PMID 22504485.
- ^ a b Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. (May 2014). "Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy". The EMBO Journal. 33 (9): 994–1010. doi:10.1002/embj.201386030. PMC 4193933. PMID 24431222.
- ^ a b Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, et al. (December 2009). "M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex". Nature Cell Biology. 11 (12): 1427–32. doi:10.1038/ncb1990. PMID 19935652.
- ^ a b c Austefjord MW, Gerdes HH, Wang X (January 2014). "Tunneling nanotubes: Diversity in morphology and structure". Communicative & Integrative Biology. 7 (1): e27934. doi:10.4161/cib.27934. PMC 3995728. PMID 24778759.
- ^ Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes HH (May 2009). "Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells". FEBS Letters. 583 (9): 1481–8. doi:10.1016/j.febslet.2009.03.065. PMID 19345217.
- ^ Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. (July 2016). "Transfer of mitochondria from astrocytes to neurons after stroke". Nature. 535 (7613): 551–5. Bibcode:2016Natur.535..551H. doi:10.1038/nature18928. PMC 4968589. PMID 27466127.
- ^ Marlein CR, Piddock RE, Mistry JJ, Zaitseva L, Hellmich C, Horton RH, et al. (May 2019). "CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma". Cancer Research. 79 (9): 2285–2297. doi:10.1158/0008-5472.CAN-18-0773. PMID 30622116.
- ^ Torralba, Daniel; Baixauli, Francesc; Sánchez-Madrid, Francisco (2016). "Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer". Frontiers in Cell and Developmental Biology. 4. doi:10.3389/fcell.2016.00107. ISSN 2296-634X. PMC 5039171. PMID 27734015.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Wang, X.; Gerdes, H.-H. (2015-07). "Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells". Cell Death & Differentiation. 22 (7): 1181–1191. doi:10.1038/cdd.2014.211. ISSN 1476-5403. PMC 4572865. PMID 25571977.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Pasquier, Jennifer; Guerrouahen, Bella S.; Al Thawadi, Hamda; Ghiabi, Pegah; Maleki, Mahtab; Abu-Kaoud, Nadine; Jacob, Arthur; Mirshahi, Massoud; Galas, Ludovic; Rafii, Shahin; Le Foll, Frank (2013-04-10). "Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance". Journal of Translational Medicine. 11 (1): 94. doi:10.1186/1479-5876-11-94. ISSN 1479-5876. PMC 3668949. PMID 23574623.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Lu, Jinjin; Zheng, Xiufen; Li, Fan; Yu, Yang; Chen, Zhong; Liu, Zheng; Wang, Zhihua; Xu, Hua; Yang, Weimin (2017-01-17). "Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells". Oncotarget. 8 (9): 15539–15552. doi:10.18632/oncotarget.14695. ISSN 1949-2553. PMC 5362504. PMID 28107184.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Li, Xiang; Zhang, Yuelin; Yeung, Sze C.; Liang, Yingmin; Liang, Xiaoting; Ding, Yue; Ip, Mary S. M.; Tse, Hung-Fat; Mak, Judith C. W.; Lian, Qizhou (2014-04-16). "Mitochondrial Transfer of Induced Pluripotent Stem Cell–Derived Mesenchymal Stem Cells to Airway Epithelial Cells Attenuates Cigarette Smoke–Induced Damage". American Journal of Respiratory Cell and Molecular Biology. 51 (3): 455–465. doi:10.1165/rcmb.2013-0529OC. ISSN 1044-1549.
- ^ Gallagher KL, Benfey PN (January 2005). "Not just another hole in the wall: understanding intercellular protein trafficking". Genes & Development. 19 (2): 189–95. doi:10.1101/gad.1271005. PMID 15655108.
- ^ Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR (June 1997). "Exchange of protein molecules through connections between higher plant plastids". Science. 276 (5321): 2039–42. doi:10.1126/science.276.5321.2039. PMID 9197266.
Further reading
- Gurke S, Barroso JF, Gerdes HH (May 2008). "The art of cellular communication: tunneling nanotubes bridge the divide". Histochemistry and Cell Biology. 129 (5): 539–50. doi:10.1007/s00418-008-0412-0. PMC 2323029. PMID 18386044.
- "Tunnelling nanotubes: Life's secret network". New Scientist. November 2008.
External links
- Hans-Hermann Gerdes Research Group - The laboratory that first observed membrane nanotubes
