Apicidin
| |
| Names | |
|---|---|
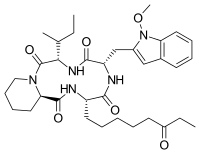
| IUPAC name
(3S,6S,9S,15aR)-9-[(2S)-2-Butanyl]-6-[(1-methoxy-1H-indol-3-yl)methyl]-3-(6-oxooctyl)octahydro-2H-pyrido[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10(3H,12H)-tetrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.163.614 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C34H49N5O6 | |
| Molar mass | 623.795 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Apicidin is a fungal metabolite, as well as a histone deacetylase inhibitor.[1]
References
