Benzo(a)pyrene
![Benzo[a]pyrene](http://upload.wikimedia.org/wikipedia/commons/thumb/f/fa/Benzo-a-pyrene.svg/200px-Benzo-a-pyrene.svg.png)
| |

| |
| Names | |
|---|---|
| IUPAC name
Benzo[a]pyrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.026 |
| KEGG | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H12 | |
| Molar mass | 252.316 g·mol−1 |
| Density | 1.24 g/cm³ (25 °C) |
| Melting point | 179 °C |
| Boiling point | 495 °C |
| 0.11 mg/L (25 °C) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzo[a]pyrene is a polycyclic aromatic hydrocarbon found in coal tar with the formula C20H12. Its metabolites are mutagenic and highly carcinogenic, and it is listed as a Group 1 carcinogen by the IARC. The compound is one of the benzopyrenes, formed by a benzene ring fused to pyrene, and is the result of incomplete combustion at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F).
Chimney sweeps suffered from scrotal cancers in the 18th century, and frequent skin cancers were noted among fuel industry workers in the 19th century. In 1933, benzo[a]pyrene was determined to be the compound responsible for these cases, and its toxicity was demonstrated when skin tumors occurred in laboratory animals repeatedly painted with coal tar. When the body attempts to metabolise benzo[a]pyrene, the resulting diol epoxide reacts and binds to DNA, resulting in mutations and eventually cancer.
Sources of Benzo[a]pyrene
Benzo[a]pyrene is found in coal tar, in automobile exhaust fumes (especially from diesel engines), in all smoke resulting from the combustion of organic material (including cigarette smoke), and in charbroiled food. Cooked meat products, regular consumption of which has been epidemiologically associated with increased levels of colon cancer[1] (although this in itself does not prove carcinogenicity),[2] have been shown to contain up to 4 ng/g of benzo[a]pyrene,[3] and up to 5.5 ng/g in fried chicken[4] and 62.6 ng/g in overcooked charcoal barbecued beef.[5]
Toxicity
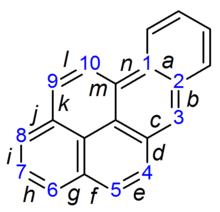
A vast number of studies over the previous three decades have documented links between benzo[a]pyrene and cancers. It has been more difficult to link cancers to specific benzo[a]pyrene sources, especially in humans, and difficult to quantify risks posed by various methods of exposure (inhalation or ingestion). Researchers at Kansas State University recently discovered a link between vitamin A deficiency and emphysema in smokers.[6] Benzo[a]pyrene was found to be behind the link, since it induces vitamin A deficiency in rats.
In 1996, a study was published that provided the clear molecular evidence conclusively linking components in tobacco smoke to lung cancer.[7] Benzo[a]pyrene, found in tobacco smoke (including cigarette smoke), was shown to cause genetic damage in lung cells that was identical to the damage observed in the DNA of most malignant lung tumours.
A 2001 National Cancer Institute study found levels of benzo[a]pyrene to be significantly higher in foods that were cooked well-done on the barbecue, particularly steaks, chicken with skin, and hamburgers. [citation needed][8] Japanese scientists showed that cooked beef contains mutagens, chemicals that are capable of altering the chemical structure of DNA [citation needed]. However, the foods themselves are not necessarily carcinogenic, even if they contain trace amounts of carcinogens, because the gastrointestinal tract protects itself against carcinomas by shedding its outer layer continuously. Furthermore, detoxification enzymes, such as cytochromes P450 have increased activities in the gut due to the normal requirement for protection from food-borne toxins. Thus in most cases small amounts of benzo[a]pyrene are metabolized by gut enzymes prior to being passed on to the blood. The lungs are not protected in either of these manners.
A recent study has found that cytochrome P450 1A1 (CYP1A1) and cytochrome P450 1B1 (CYP1B1) are both protective and, confusingly, necessary for benzo[a]pyrene toxicity. Experiments with strains of mice engineered to remove (knockout) CYP1A1 and CYP1B1 reveal that CYP1A1 primarily acts to protect mammals from low doses of benzo[a]pyrene, and that removing this protection causes the biological accumulation of large concentrations of benzo[a]pyrene. Unless CYP1B1 is also knocked out, benzo[a]pyrene toxicity results from the bioactivation of benzo[a]pyrene to the ultimate toxic compound, benzo[a]pyrene -7,8-dihydrodiol-9,10-epoxide (see below).[9]
Interaction with DNA

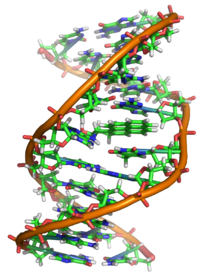
Properly speaking, benzo[a]pyrene is a procarcinogen, meaning that the mechanism of carcinogenesis of benzo[a]pyrene depends on enzymatic metabolism of benzo[a]pyrene to the ultimate mutagen, benzo[a]pyrene diol epoxide, pictured above at right. This molecule intercalates in DNA, covalently bonding to the nucleophilic guanine nucleobases at the N2 position. X-ray crystallographic and nuclear magnetic resonance structure studies show that this binding distorts the DNA,[11] inducing mutations by perturbing the double-helical DNA structure. This disrupts the normal process of copying DNA and induces mutations, which explains the occurrence of cancer after exposure. This mechanism of action is similar to that of aflatoxin which binds to the N7 position of guanine.[12]
There are indications that benzo[a]pyrene diol epoxide specifically targets the protective p53 gene.[13] This gene is a transcription factor that regulates the cell cycle and hence functions as a tumor suppressor. By inducing G (guanine) to T (thymidine) transversions in transversion hotspots within p53, there is a probability that benzo[a]pyrene diol epoxide inactivates the tumor suppression ability in certain cells, leading to cancer.
Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is the carcinogenic product of three enzymatic reactions:[14]
- Benzo[a]pyrene is first oxidized by cytochrome P4501A1 to form a variety of products, including (+)benzo[a]pyrene-7,8-epoxide.[15]
- This product is metabolized by epoxide hydrolase, opening up the epoxide ring to yield (-)benzo[a]pyrene-7,8-dihydrodiol.
- The ultimate carcinogen is formed after another reaction with cytochrome P4501A1 to yield the (+)benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. It is this diol epoxide that covalently binds to DNA.
Benzo[a]pyrene induces cytochrome P4501A1 (CYP1A1) by binding to the AHR (aryl hydrocarbon receptor) in the cytosol.[16] Upon binding the transformed receptor translocates to the nucleus where it dimerises with ARNT (aryl hydrocarbon receptor nuclear translocator) and then binds xenobiotic response elements (XREs) in DNA located upstream of certain genes. This process increases transcription of certain genes, notably CYP1A1, followed by increased CYP1A1 protein production.[16] This process is similar to induction of CYP1A1 by certain polychlorinated biphenyls and dioxins. Seemingly, CYP1A1 activity in the intestinal mucosa prevents major amounts of ingested benzo(a)pyrene to enter portal blood and systemic circulation.[17] Intestinal, but not hepatic, expression of CYP1A1 depends on TOLL-like receptor 2 (TLR2),[18] which is a eucaryotic receptor for bacterial surface structures such as lipoteichoic acid.
Moreover, benzo[a]pyrene has been found to activate a transposon, LINE1, in humans.[19]
See also
- Benzopyrene
- Benzo[e]pyrene
- Pyrene, a four-ring analogue
- Toxification
References
- ^ Le Marchand L, Hankin JH, Pierce LM, Sinha R, Nerurkar PV, Franke AA, Wilkens LR, Kolonel LN, Donlon T, Seifried A, Custer LJ, Lum-Jones A, Chang W. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutation Research. 2002 Sep 30;506-507:205-14. PMID 12351160
- ^ Truswell AS. Meat consumption and cancer of the large bowel. European Journal of Clinical Nutrition. 2002 Mar;56 Suppl 1:S19-24. PMID 11965518
- ^ Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food and Chemical Toxicology 2002;40(1):133. doi:10.1016/S0278-6915(00)00158-7
- ^ Lee BM, Shim GA. Dietary exposure estimation of benzo[a]pyrene and cancer risk assessment. Journal of Toxicology and Environmental Health Part A. 2007 Aug;70(15-16):1391-4. PMID 17654259
- ^ Aygün SF, Kabadayi F. Determination of benzo[a]pyrene in charcoal grilled meat samples by HPLC with fluorescence detection. International Journal of Food Sciences and Nutrition. 2005 Dec;56(8):581-5. PMID 16638662
- ^ "Benzopyrene and Vitamin A deficiency". Researcher links cigarettes, vitamin A and emphysema. Retrieved March 5, 2005.
- ^ Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996 October 18;274(5286):430-2.
- ^ http://cebp.aacrjournals.org/content/14/8/2030.full
- ^ Data presented by Daniel W. Nebert in research seminars 2007
- ^ Created from PDB 1JDG
- ^ Volk DE, Thiviyanathan V, Rice JS, Luxon BA, Shah JH, Yagi H, Sayer JM, Yeh HJ, Jerina DM, Gorenstein DG. Solution structure of a cis-opened (10R)-N6-deoxyadenosine adduct of (9S,10R)-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in a DNA duplex. Biochemistry. 2003 February 18;42(6):1410-20.
- ^ Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol. 1994;34:135-72.
- ^ Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002 October 21;21(48):7435-51.
- ^ Hao Jiang, Stacy L. Gelhaus, Dipti Mangal, Ronald G. Harvey, Ian A. Blair, Trevor M. Penning: Metabolism of Benzo[a]pyrene in Human Bronchoalveolar H358 Cells Using Liquid Chromatography-Mass Spectrometry, Chem. Res. Toxicol., 2007, 20 (9), pp 1331–1341 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2423818/
- ^ Shou M, Gonzalez FJ, Gelboin HV. Stereoselective epoxidation and hydration at the K-region of polycyclic aromatic hydrocarbons by cDNA-expressed cytochromes P450 1A1, 1A2, and epoxide hydrolase. Biochemistry. 1996 December 10;35(49):15807-13
- ^ a b Whitlock, JP Jr. (April 1999). "Induction of cytochrome P4501A1". Annual Review of Pharmacology and Toxicology. 39: 103–125. doi:10.1146/annurev.pharmtox.39.1.103. PMID 10331078.
{{cite journal}}:|access-date=requires|url=(help); Cite has empty unknown parameter:|coauthors=(help) - ^ Uno, S.; Dragin, N; Miller, ML; Dalton, TP; et al. (2008). "Basal and inducible CYP1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract". Free Radic Biol Med. 44 (4): 570–83. doi:10.1016/j.freeradbiomed.2007.10.044. PMID 17997381.
{{cite journal}}: Explicit use of et al. in:|first4=(help) - ^ Do, KN; Fink, LN; Jensen, TE; Gautier, L; Parlesak, A (2012). "TLR2 controls intestinal carcinogen detoxication by CYP1A1". PLoS ONE. 7 (3): e32309. doi:10.1371/journal.pone.0032309.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Vilius Stribinskis and Kenneth S. Ramos (2006). Activation of Human Long Interspersed Nuclear Element 1 Retrotransposition by Benzo(a)pyrene, a Ubiquitous Environmental Carcinogen. Cancer Res 2006; 66: (5).
External links
- International Chemical Safety Card 0104
- National Pollutant Inventory - Polycyclic Aromatic Hydrocarbon Fact Sheet
- "Benzopyrene in Barbeque". Newhouse A1. Retrieved March 5, 2005. [dead link]-->
- "Lung cancer as consequence by Benzopyrene in [[smoke]]rs". Lung Cancer. Retrieved March 5, 2005.
{{cite web}}: URL–wikilink conflict (help) - "Levels of Benzopyrene in Burnt toasts". Guardian Unlimited. Retrieved March 5, 2005.
{{cite web}}: Text "Close encounters" ignored (help); Text "Special reports" ignored (help) - "DNA interaction with Benzopyrene". DNA. Retrieved March 5, 2005. [dead link]
- "Crystal and molecular structure of a benzo-a-pyrene 7,8-diol 9,10-epoxide N2-deoxyguanosine adduct: Absolute configuration and conformation". Proceedings of the National Academy of Sciences. Retrieved January 3, 2006.
