Cyanoacrylate
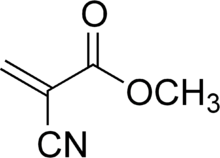
Cyanoacrylate is the generic name for a family of fast-acting adhesives with industrial, medical and household uses. They include methyl 2-cyanoacrylate, ethyl-2-cyanoacrylate (commonly sold under trade names like "Super Glue" and "Krazy Glue"), and n-butyl cyanoacrylate (used in veterinary and skin glues). The related compound 2-octyl cyanoacrylate is a medical grade glue; it was developed to be non-toxic and less irritating to skin tissue. Cyanoacrylate adhesives are sometimes known as instant glues. The abbreviation "CA" is commonly used for industrial grades.
Development

The original cyanoacrylates (the chemical name for the glue) were discovered in 1942 in a search for materials to make clear plastic gun sights for the war, when scientists stumbled upon a formulation that stuck to everything that it came in contact with.[1] However, cyanoacrylates were quickly rejected by the American researchers precisely because they stuck to everything. In 1951, cyanoacrylates were rediscovered by Eastman Kodak researchers Harry Coover and Fred Joyner, who recognized their true commercial potential, and it was first sold as a commercial product "Eastman #910" (later "Eastman 910") in 1958.
During the 1960s, Eastman Kodak sold cyanoacrylate raw feedstock to Loctite, which in turn repackaged and distributed it under a different brand name "Loctite Quick Set 404." In 1971, Loctite developed its own manufacturing technology and introduced its own line of cyanoacrylate, called "Super Bonder". Loctite quickly gained market share, and by late 1970s it was believed to have exceeded Eastman Kodak's share in the North American industrial cyanoacrylate market. Other manufacturers of cyanoacrylate included Permabond Division of National Starch and Chemical, Inc., which was a subsidiary of Unilever. Together, Loctite, Eastman and Permabond accounted for approximately 75% of the industrial cyanoacrylate market.[2]
Properties
In its liquid form, cyanoacrylate consists of monomers of cyanoacrylate molecules. Methyl-2-cyanoacrylate (CH2=C(CN)COOCH3 or C5H5NO2) has a molecular weight equal to 111.1, a flashpoint of 79 °C, and 1.1 times the density of water.[3] Ethyl-2-cyanoacrylate (C6H7NO2) has a molecular weight equal to 125 and a flashpoint of >75°C. To facilitate easy handling, a cyanoacrylate adhesive is frequently formulated with an ingredient such as fumed silica to make it more viscous or gel-like. More recently, formulations are available with additives to increase shear strength, creating a more impact resistant bond. Such additives may include rubber, as in Loctite's Ultra Gel, and/or unspecified additives, as in Instant Krazy Glue's ADVANCED Formula.
In general, cyanoacrylate is an acrylic resin that rapidly polymerises in the presence of water (specifically hydroxide ions), forming long, strong chains, joining the bonded surfaces together. Because the presence of moisture causes the glue to set, exposure to moisture in the air can cause a tube or bottle of glue to become unusable over time. To prevent an opened container of glue from setting before use, it must be stored in an airtight jar or bottle with a package of silica gel. Another tactic is attaching a hypodermic needle on the opening of glue. After applying, residual glue soon clogs the needle, keeping moisture out. The clog is removed by heating the needle (e.g., by a lighter) before use.

Uses
Cyanoacrylate glue has a low shearing strength, which has also led to its use as a temporary adhesive in cases where the piece can easily be sheared off at a later time. Common examples include mounting a workpiece to a sacrificial glue block on a lathe, and tightening pins and bolts.
Cyanoacrylates are used to assemble prototype electronics (see wire wrap), flying model aircraft, and as retention dressings for nuts and bolts. Their effectiveness in bonding metal and general versatility have also made them popular among modeling and miniatures hobbyists. They are used to re-harden the boxes and shanks of ballerinas' pointe shoes as well.
Cyanoacrylate glue's ability to resist water has made it popular with marine aquarium hobbyists for fragging corals. The cut branches of hard corals such as Acropora can be glued to a piece of live rock (harvested reef coral) or Milliput (epoxy putty) to allow the new frag to grow out. It is actually safe to use directly in the tank, unlike silicone, which must be cured to be safe. However, as a class of adhesives, traditional cyanoacrylates are classified as having "weak" resistance to both moisture and heat[4] although the inclusion of phthalic anhydride reportedly counteracts both of these characteristics.[5]
In most cases, standard cyanoacrylate adhesive does not functionally bond well with smooth glass, although it can be used as a quick, temporary bond prior to application of a proper glass epoxy or cyanoacrylate specifically formulated for use on glass.[6] A mechanical adhesive bond may be formed around glass fibre mat or tissue to reinforce joints or to fabricate small parts.
When added to baking soda (sodium bicarbonate), cyanoacrylate glue forms a hard, lightweight filler/adhesive (baking soda is first used to fill a gap then the adhesive is dropped onto the baking soda). This works well with porous materials that the glue does not work well with alone. This method is sometimes used by aircraft modelers to assemble or repair polystyrene foam parts. It is also used to repair small nicks in the leading edge of composite propeller blades on light aircraft. Note that the reaction between cyanoacrylate and baking soda is very exothermic (heat-producing) and also produces noxious vapors. See Reaction with cotton below.
Cyanoacrylate is used as a forensic tool to capture latent fingerprints on non-porous surfaces like glass, plastic, etc.[7] Cyanoacrylate is warmed to produce fumes that react with the invisible fingerprint residues and atmospheric moisture to form a white polymer (polycyanoacrylate) on the fingerprint ridges. The ridges can then be recorded. The developed fingerprints are, on most surfaces (except on white plastic or similar), visible to the naked eye. Invisible or poorly visible prints can be further enhanced by applying a luminescent or non-luminescent stain.
Thin CA glue also has application in woodworking. It can be used as a fast drying, glossy finish. The use of oil (such as boiled linseed oil) may be used to control the rate at which the CA cures. CA glue is also used in combination with sawdust (from a saw or sanding) to fill voids and cracks. These repair methods are used on piano soundboards, wood instruments, and wood furniture.
Some rock climbers use cyanoacrylate to repair damage to the skin on their fingertips.[8][9] Similarly, stringed-instrument players can form protective finger caps (in addition to calluses) with cyanoacrylates.
CA glue was in veterinary use for mending bone, hide, and tortoise shell by at least the early 1970s. The inventor of cyanoacrylates, Harry Coover, said in 1966 that a CA spray was used in the Vietnam War to retard bleeding in wounded soldiers until they could be brought to a hospital. Butyl cyanoacrylate has been used medically since the 1970s outside the US, but, due to its potential to irritate the skin, the U.S. Food and Drug Administration did not approve its use as a medical adhesive until 1998 with Dermabond.[10] Research has demonstrated the use of cyanoacrylate in wound closure as being safer and more functional than traditional suturing (stitches).[11] The adhesive has demonstrated superior performance in the time required to close a wound, incidence of infection (suture canals through the skin's epidermal, dermal, and subcutaneous fat layers introduce extra routes of contamination),[11] and final cosmetic appearance.[12][13]
While standard 'superglue' is 100% ethyl cyanoacrylate, many custom formulations (e.g.,, 91% ECA, 9% poly(methyl methacrylate), <0.5% hydroquinone, and a small amount of organic sulfonic acid[14] and variations on the compound N-butyl-cyanoacrylate's for medical applications[11]) have come to be used for specific applications.
Cyanoacrylate is used in Archery to glue fletching to arrow shafts. The special 'fletch-tite' glues are really Cyanoacrylate repackaged in special fletching glue kits. Often these tubes have a long thin metal nozzle to aid in better accuracy in the application of the glue to the base of the feather or plastic fletching to ensure a good bond to the arrow shaft.
Cyanoacrylate is used in the cosmetology / beauty industry as an adhesive for some artificial nail enhancements such as nail tips and nail wraps.[citation needed]
Safety issues
Toxicity
The fumes from CA are a vaporized form of the cyanoacrylate monomer that irritate sensitive membranes in the eyes, nose, and throat. They are immediately polymerized by the moisture in the membranes and become inert. These risks can be minimized by using CA in well ventilated areas. About 5% of the population can become sensitized to CA fumes after repeated exposure, resulting in flu-like symptoms.[15] It may also act as a skin irritant and may cause an allergic skin reaction. The ACGIH assign a Threshold Limit Value exposure limit of 200 parts per billion. On rare occasions, inhalation may trigger asthma. There is no singular measurement of toxicity for all cyanoacrylate adhesives as there is a wide variety of adhesives that contain various cyanoacrylate formulations.
The United States National Toxicology Program and the United Kingdom Health and Safety Executive have concluded that the use of ethyl cyanoacrylate is safe and that additional study is unnecessary.[16] 2-octyl cyanoacrylate degrades much more slowly due to its longer organic backbone that slows the degradation of the adhesive enough to remain below the threshold of tissue toxicity. Due to the toxicity issues of ethyl cyanoacrylate, the use of 2-octyl cyanoacrylate for sutures is preferred.
Reaction with cotton
Applying cyanoacrylate to materials made of cotton or wool (such as cotton swabs, cotton balls, and certain yarns or fabrics) results in a powerful, rapid exothermic reaction. The heat released may cause serious burns,[17] ignite the cotton product, or release irritating white smoke. Material Safety Data Sheets for cyanoacrylate instruct users not to wear cotton or wool clothing, especially cotton gloves, when applying or handling cyanoacrylates.[18]
Solvents and debonders
Acetone, commonly found in nail polish remover, is a widely available solvent capable of softening cured cyanoacrylate.[19] Other solvents include Nitromethane, Dimethyl sulfoxide, and Methylene chloride, although the latter two of these solvents are toxic.[20] Gamma-butyrolactone may also be used to remove cured cyanoacrylate.[21] Commercial debonders are also available.[22]
See also
- Methyl cyanoacrylate
- Ethyl cyanoacrylate
- Butyl cyanoacrylate
- Octyl cyanoacrylate
- 2-Octyl cyanoacrylate
References
- ^ "Inventor of the Week Archive". Lemelson-MIT Program. September 2004. Retrieved 13 February 2010.
- ^ HBS, “Loctite Corporation: Industrial Product Group,” July 15, 1991, p.3
- ^ http://www.inchem.org/documents/icsc/icsc/eics1272.htm
- ^ Petrie, Edward M. (2000). Handbook of adhesives and sealants. New York, NY: McGraw-Hill. p. 354. ISBN 0-07-049888-1.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Petrie, Edward M. (2000). Handbook of adhesives and sealants. New York, NY: McGraw-Hill. p. 389. ISBN 0-07-049888-1.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ http://nautarch.tamu.edu/crl/conservationmanual/File5.htm
- ^ Eric W. Brown "The Cyanoacrylate Fuming Method"
- ^ "Bouldering". climbingaction.com. Retrieved 2011-02-19.
- ^ Anahad O'Connor (4 December 2007). "The Claim: Super Glue Can Heal Wounds". The New York Times. Retrieved 19 February 2011.
- ^ Singer, A. J.; McClain, S. A.; Katz, A. (2004). "A porcine epistaxis model: hemostatic effects of octylcyanoacrylate". Otolaryngology-Head and Neck Surgery. 130 (5): 553–557. doi:10.1016/j.otohns.2003.09.035. PMID 15138419.
- ^ a b c http://www.jpgmonline.com/article.asp?issn=0022-3859;year=1986;volume=32;issue=2;spage=97;epage=100;aulast=Dalvi
- ^ Fischl, R.A.: An adhesive for primary closure of skin incisions: a preliminary report. Plast. Reconstr. Surg. 30: 607-610, 1962
- ^ Rothnie, N.G. and Taylor, G. W. Sutureless skin closure-a clinical trial. Brit. Med. J. 2: 1027-1030, 1963
- ^ Safety data for ethyl cyanoacrylate from the Physical and Theoretical Chemistry Laboratory of Oxford University
- ^ CA PLUS Adhesives, Inc. FAQ
- ^ Methyl Cyanoacrylate and Ethyl Cyanoacrylate from inchem.org
- ^ Clarke, TFE (2011). "Superglue (Cyanoacrylate) in the Nose". Journal of Plastic, Reconstructive & Aesthetic Surgery. 64 (7): e170–3. doi:10.1016/j.bjps.2011.03.009. PMID 21481658.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help) - ^ "Material Safety Data Sheet" (PDF). accumetricinc.com. Retrieved 2008-06-09.
- ^ Moschos, M.; Droutsas, D.; Boussalis, P.; Tsioulias, G. (1997). "Clinical experience with cyanoacrylate tissue adhesive". Documenta Ophthalmologica. 93 (3). Soringer: 237–245. doi:10.1007/BF02569064. PMID 9550352. Retrieved 29 March 2011.
- ^ Duvvi, Sham K.; Lo, Stephen; Kumar, R; Spraggs, P (2005). "Superglue (Cyanoacrylate) in the Nose". Otolaryngol Head and Neck Surgery. 133 (5): 803–804. doi:10.1016/j.otohns.2004.09.090. Retrieved 29 March 2011.
- ^ Shantha, K.L.; Krishnamurti, N.; Krishnamurti, N. (1989). "Developments and applications of cyanoacrylate adhesives". Journal of Adhesion Science and Technology. 3 (1). VSP: 237–260. doi:10.1163/156856189X00191. Retrieved 29 March 2011.
- ^ "Product Description". 3M. Retrieved 27 March 2011.
Further reading
- derma+flex® QS™ 510k Letter: http://www.accessdata.fda.gov/cdrh_docs/pdf10/K101276.pdf
- LiquiBand® 510k Letter: http://www.accessdata.fda.gov/cdrh_docs/pdf8/K083531.pdf
- Fernandez, Tania; Bliskovsky, Val (2 January 2003). "Cyanoacrylate Technology: Stay Glued". Pharmbiz.com.
{{cite web}}: Missing or empty|url=(help); Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - Hayes, Sharon Caskey (11 July 2004). "Discovery of Super Glue helped land Coover in National Inventors Hall of Fame". Kingsport Times-News.
- Jueneman, F. (1981). "Stick it to um". Industrial Research & Development. p. 19.
{{cite news}}: Unknown parameter|month=ignored (help) - Perry, L. C. "An evaluation of acute incisional strength with Traumaseal surgical tissue adhesive wound closure". Dimensional Analysis Systems Inc.
- Quinn, J.; Kissack, J. (1994). "Tissue Adhesives for Laceration Repair During Sporting Events". Clinical Journal of Sports Medicine. 4 (4): 245. doi:10.1097/00042752-199410000-00006.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - Schwade, Nathan D. (10 April 2002). "Wound Adhesives, 2-Octyl Cyanoacrylate". eMedicine article.
- Vinters, H. V.; Galil, K. A.; Lundie, M. J.; Kaufmann, J. C. (1985). "The histotoxicity of cyanoacrylates. A selective review". Neuroradiology. 27 (4): 279–291. doi:10.1007/BF00339559. PMID 3900798.
External links
- Was Super Glue invented to seal battle wounds in Vietnam? (from The Straight Dope)
- Cyanoacrylate Toxicity
- Cyanoacrylate Adhesive / Super Glue Safety Data Sheets
- Safety in the Home: Super Glue - Queensland Health
- Cyanoacrylate Technical Data Sheet
- U.S. patent 2,768,109 Alcohol-Catalyzed α-Cyanoacrylate Adhesive Compositions, filed June 1954, issued October 1956.
