Glucagon
| glucagon | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | GCG(53-81)glucagoneglucagon recombinant | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | GeneCards: [1]; OMA:- orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body.[1] It is also used as a medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose.[2] It is produced from proglucagon, encoded by the GCG gene.
The pancreas releases glucagon when the amount of glucose in the bloodstream is too low. Glucagon causes the liver to engage in glycogenolysis: converting stored glycogen into glucose, which is released into the bloodstream.[3] High blood-glucose levels, on the other hand, stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues. Thus, glucagon and insulin are part of a feedback system that keeps blood glucose levels stable. Glucagon increases energy expenditure and is elevated under conditions of stress.[4] Glucagon belongs to the secretin family of hormones.
Structure
[edit]Glucagon is a 29-amino acid polypeptide. Its primary structure in humans is: NH2-His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr-COOH (HSQGTFTSDYSKYLDSRRAQDFVQWLMNT).
The polypeptide has a molecular mass of 3485 daltons.[5] Glucagon is a peptide (nonsteroid) hormone.
Physiology
[edit]Production
[edit]
The hormone is synthesized and secreted from alpha cells (α-cells) of the islets of Langerhans, which are located in the endocrine portion of the pancreas. Glucagon is produced from the preproglucagon gene Gcg. Preproglucagon first has its signal peptide removed by signal peptidase, forming the 160-amino acid protein proglucagon.[6] Proglucagon is then cleaved by proprotein convertase 2 to glucagon (amino acids 33-61) in pancreatic islet α cells. In intestinal L cells, proglucagon is cleaved to the alternate products glicentin (1–69), glicentin-related pancreatic polypeptide (1–30), oxyntomodulin (33–69), glucagon-like peptide 1 (72–107 or 108), and glucagon-like peptide 2 (126–158).[6]
In rodents, the alpha cells are located in the outer rim of the islet. Human islet structure is much less segregated, and alpha cells are distributed throughout the islet in close proximity to beta cells. Glucagon is also produced by alpha cells in the stomach.[7]
Recent research has demonstrated that glucagon production may also take place outside the pancreas, with the gut being the most likely site of extrapancreatic glucagon synthesis.[8]
Regulation
[edit]Production, which is otherwise freerunning, is suppressed/regulated by amylin, a peptide hormone co-secreted with insulin from the pancreatic β cells.[9] As plasma glucose levels recede, the subsequent reduction in amylin secretion alleviates its suppression of the α cells, allowing for glucagon secretion.
Secretion of glucagon is stimulated by:
- Hypoglycemia
- Epinephrine (via β2, α2,[10] and α1[11] adrenergic receptors)
- Arginine
- Alanine (often from muscle-derived pyruvate/glutamate transamination (see alanine transaminase reaction).
- Acetylcholine[12]
- Cholecystokinin
- Gastric inhibitory polypeptide
- Gastrin[13]
Secretion of glucagon is inhibited by:
- Somatostatin
- Amylin[9]
- Insulin (via GABA)[14]
- PPARγ/retinoid X receptor heterodimer.[15]
- Increased free fatty acids and keto acids into the blood.[16]
- Increased urea production
- Glucagon-like peptide-1
Function
[edit]Glucagon generally elevates the concentration of glucose in the blood by promoting gluconeogenesis and glycogenolysis.[17] Glucagon also decreases fatty acid synthesis in adipose tissue and the liver, as well as promoting lipolysis in these tissues, which causes them to release fatty acids into circulation where they can be catabolised to generate energy in tissues such as skeletal muscle when required.[18]
Glucose is stored in the liver in the form of the polysaccharide glycogen, which is a glucan (a polymer made up of glucose molecules). Liver cells (hepatocytes) have glucagon receptors. When glucagon binds to the glucagon receptors, the liver cells convert the glycogen into individual glucose molecules and release them into the bloodstream, in a process known as glycogenolysis. As these stores become depleted, glucagon then encourages the liver and kidney to synthesize additional glucose by gluconeogenesis. Glucagon turns off glycolysis in the liver, causing glycolytic intermediates to be shuttled to gluconeogenesis.
Glucagon also regulates the rate of glucose production through lipolysis. Glucagon induces lipolysis in humans under conditions of insulin suppression (such as diabetes mellitus type 1).[19]
Glucagon production appears to be dependent on the central nervous system through pathways yet to be defined. In invertebrate animals, eyestalk removal has been reported to affect glucagon production. Excising the eyestalk in young crayfish produces glucagon-induced hyperglycemia.[20]
Mechanism of action
[edit]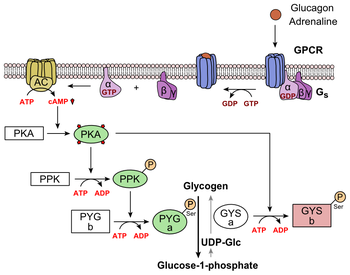
Glucagon binds to the glucagon receptor, a G protein-coupled receptor, located in the plasma membrane of the cell. The conformation change in the receptor activates a G protein, a heterotrimeric protein with αs, β, and γ subunits. When the G protein interacts with the receptor, it undergoes a conformational change that results in the replacement of the GDP molecule that was bound to the α subunit with a GTP molecule.[21] This substitution results in the releasing of the α subunit from the β and γ subunits. The alpha subunit specifically activates the next enzyme in the cascade, adenylate cyclase.
Adenylate cyclase manufactures cyclic adenosine monophosphate (cyclic AMP or cAMP), which activates protein kinase A (cAMP-dependent protein kinase). This enzyme, in turn, activates phosphorylase kinase, which then phosphorylates glycogen phosphorylase b (PYG b), converting it into the active form called phosphorylase a (PYG a). Phosphorylase a is the enzyme responsible for the release of glucose 1-phosphate from glycogen polymers. An example of the pathway would be when glucagon binds to a transmembrane protein. The transmembrane proteins interacts with Gɑβ𝛾. Gαs separates from Gβ𝛾 and interacts with the transmembrane protein adenylyl cyclase. Adenylyl cyclase catalyzes the conversion of ATP to cAMP. cAMP binds to protein kinase A, and the complex phosphorylates glycogen phosphorylase kinase.[22] Phosphorylated glycogen phosphorylase kinase phosphorylates glycogen phosphorylase. Phosphorylated glycogen phosphorylase clips glucose units from glycogen as glucose 1-phosphate.
Additionally, the coordinated control of glycolysis and gluconeogenesis in the liver is adjusted by the phosphorylation state of the enzymes that catalyze the formation of a potent activator of glycolysis called fructose 2,6-bisphosphate.[23] The enzyme protein kinase A (PKA) that was stimulated by the cascade initiated by glucagon will also phosphorylate a single serine residue of the bifunctional polypeptide chain containing both the enzymes fructose 2,6-bisphosphatase and phosphofructokinase-2. This covalent phosphorylation initiated by glucagon activates the former and inhibits the latter. This regulates the reaction catalyzing fructose 2,6-bisphosphate (a potent activator of phosphofructokinase-1, the enzyme that is the primary regulatory step of glycolysis)[24] by slowing the rate of its formation, thereby inhibiting the flux of the glycolysis pathway and allowing gluconeogenesis to predominate. This process is reversible in the absence of glucagon (and thus, the presence of insulin).
Glucagon stimulation of PKA inactivates the glycolytic enzyme pyruvate kinase,[25] inactivates glycogen synthase,[26] and activates hormone-sensitive lipase,[27] which catabolizes glycerides into glycerol and free fatty acid(s), in hepatocytes.
Glucagon also inactivates acetyl-CoA carboxylase, which creates malonyl-CoA from acetyl-CoA, through cAMP-dependent and/or cAMP-independent kinases.[28]
Malonyl-CoA is a product formed by ACC during denovo synthesis and an allosteric inhibitor of Carnitine palmitoyltransferase I (CPT1), a mitochondrial enzyme important for bringing fatty acids into the intermembrane space of the mitochondria for β-oxidation.[29] Glucagon decreases malonyl-CoA through inhibition of acetyl-CoA carboxylase and through reduced glycolysis through its aforementioned reduction in Fructose 2,6-bisphosphate. Thus, reduction in malonyl-CoA is a common regulator for the increased fatty acid metabolism effects of glucagon.
Pathology
[edit]Abnormally elevated levels of glucagon may be caused by pancreatic tumors, such as glucagonoma, symptoms of which include necrolytic migratory erythema,[30] reduced amino acids, and hyperglycemia. It may occur alone or in the context of multiple endocrine neoplasia type 1.[31]
Elevated glucagon is the main contributor to hyperglycemic ketoacidosis in undiagnosed or poorly treated type 1 diabetes. As the beta cells cease to function, insulin and pancreatic GABA are no longer present to suppress the freerunning output of glucagon. As a result, glucagon is released from the alpha cells at a maximum, causing a rapid breakdown of glycogen to glucose and fast ketogenesis .[32] It was found that a subset of adults with type 1 diabetes took 4 times longer on average to approach ketoacidosis when given somatostatin (inhibits glucagon production) with no insulin.[citation needed] Inhibiting glucagon has been a popular idea of diabetes treatment, however, some have warned that doing so will give rise to brittle diabetes in patients with adequately stable blood glucose.[citation needed]
The absence of alpha cells (and hence glucagon) is thought to be one of the main influences in the extreme volatility of blood glucose in the setting of a total pancreatectomy.
History
[edit]In the early 1920s, several groups noted that pancreatic extracts injected into diabetic animals would result in a brief increase in blood sugar prior to the insulin-driven decrease in blood sugar.[6] In 1922, C. Kimball and John R. Murlin identified a component of pancreatic extracts responsible for this blood sugar increase, terming it "glucagon", a portmanteau of "glucose agonist".[6][33] In the 1950s, scientists at Eli Lilly isolated pure glucagon, crystallized it, and determined its amino acid sequence.[6][34][35] This led to the development of the first radioimmunoassay for detecting glucagon, described by Roger Unger's group in 1959.[6]
A more complete understanding of its role in physiology and disease was not established until the 1970s, when a specific radioimmunoassay was developed.[36]
In 1979, while working in Joel Habener's laboratory at Massachusetts General Hospital, Richard Goodman collected islet cells from Brockman bodies of American anglerfish in order to investigate somatostatin.[37] By splicing DNA from anglerfish islet cells into bacteria, Goodman was able to identify the gene which codes for somatostatin.[37] P. Kay Lund joined the Habener lab and used Goodman's bacteria to search for the gene for glucagon.[37] In 1982, Lund and Goodman published their discovery that the proglucagon gene codes for three distinct peptides: glucagon and two novel peptides.[37] Graeme Bell at Chiron Corporation led a team which isolated the two latter peptides, which are now known as glucagon-like peptide-1 and glucagon-like peptide-2.[37] This opened the door to the discovery of the glucagon-like peptide-1 receptor and then drugs which target that receptor, known as GLP-1 receptor agonists.[37]
See also
[edit]References
[edit]- ^ Voet D, Voet JG (2011). Biochemistry (4th ed.). New York: Wiley.
- ^ Reece J, Campbell N (2002). Biology. San Francisco: Benjamin Cummings. ISBN 978-0-8053-6624-2.
- ^ Orsay J (2014). Biology 1: Molecules. Examkrackers Inc. p. 77. ISBN 978-1-893858-70-1.
- ^ Jones BJ, Tan T, Bloom SR (March 2012). "Minireview: Glucagon in stress and energy homeostasis". Endocrinology. 153 (3): 1049–54. doi:10.1210/en.2011-1979. PMC 3281544. PMID 22294753.
- ^ Unger RH, Orci L (June 1981). "Glucagon and the A cell: physiology and pathophysiology (first two parts)". The New England Journal of Medicine. 304 (25): 1518–24. doi:10.1056/NEJM198106183042504. PMID 7015132.
- ^ a b c d e f Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH (April 2017). "The New Biology and Pharmacology of Glucagon". Physiol Rev. 97 (2): 721–766. doi:10.1152/physrev.00025.2016. PMID 28275047.
- ^ Unger RH, Cherrington AD (January 2012). "Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover". The Journal of Clinical Investigation. 122 (1): 4–12. doi:10.1172/JCI60016. PMC 3248306. PMID 22214853.
- ^ Holst JJ, Holland W, Gromada J, Lee Y, Unger RH, Yan H, Sloop KW, Kieffer TJ, Damond N, Herrera PL (April 2017). "Insulin and Glucagon: Partners for Life". Endocrinology. 158 (4): 696–701. doi:10.1210/en.2016-1748. PMC 6061217. PMID 28323959.
- ^ a b Zhang XX, Pan YH, Huang YM, Zhao HL (May 2016). "Neuroendocrine hormone amylin in diabetes". World Journal of Diabetes. 7 (9): 189–197. doi:10.4239/wjd.v7.i9.189. PMC 4856891. PMID 27162583.
- ^ Layden BT, Durai V, Lowe WL (2010). "G-Protein-Coupled Receptors, PANCREATIC Islets, and DiAbetes". Nature Education. 3 (9): 13.
- ^ Skoglund G, Lundquist I, Ahrén B (November 1987). "Alpha 1- and alpha 2-adrenoceptor activation increases plasma glucagon levels in the mouse". European Journal of Pharmacology. 143 (1): 83–8. doi:10.1016/0014-2999(87)90737-0. PMID 2891547.
- ^ Honey RN, Weir GC (October 1980). "Acetylcholine stimulates insulin, glucagon, and somatostatin release in the perfused chicken pancreas". Endocrinology. 107 (4): 1065–8. doi:10.1210/endo-107-4-1065. PMID 6105951.
- ^ Rehfeld JF, Holst JJ, Kühl C (February 1978). "The effect of gastrin on basal and aminoacid-stimulated insulin and glucagon secretion in man". European Journal of Clinical Investigation. 8 (1): 5–9. doi:10.1111/j.1365-2362.1978.tb00800.x. PMID 417933. S2CID 38154468.
- ^ Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q (January 2006). "Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system". Cell Metabolism. 3 (1): 47–58. doi:10.1016/j.cmet.2005.11.015. PMID 16399504.
- ^ Krätzner R, Fröhlich F, Lepler K, Schröder M, Röher K, Dickel C, Tzvetkov MV, Quentin T, Oetjen E, Knepel W (February 2008). "A peroxisome proliferator-activated receptor gamma-retinoid X receptor heterodimer physically interacts with the transcriptional activator PAX6 to inhibit glucagon gene transcription". Molecular Pharmacology. 73 (2): 509–17. doi:10.1124/mol.107.035568. PMID 17962386. S2CID 10108970.
- ^ Johnson LR (2003). Essential Medical Physiology. Academic Press. pp. 643–. ISBN 978-0-12-387584-6.
- ^ Voet D, Voet JG (2011). Biochemistry (4th ed.). New York: Wiley.
- ^ Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH (December 2010). "The metabolic actions of glucagon revisited". Nature Reviews. Endocrinology. 6 (12): 689–697. doi:10.1038/nrendo.2010.187. PMC 3563428. PMID 20957001.
- ^ Liljenquist JE, Bomboy JD, Lewis SB, Sinclair-Smith BC, Felts PW, Lacy WW, Crofford OB, Liddle GW (January 1974). "Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men". The Journal of Clinical Investigation. 53 (1): 190–7. doi:10.1172/JCI107537. PMC 301453. PMID 4808635.
- ^ Leinen RL, Giannini AJ (1983). "Effect of eyestalk removal on glucagon induced hyperglycemia in crayfish". Society for Neuroscience Abstracts. 9: 604.
- ^ "Glucagon Signaling Pathway". News-Medical.net. 2018-03-01. Retrieved 2021-03-30.
- ^ Yu Q, Shuai H, Ahooghalandari P, Gylfe E, Tengholm A (July 2019). "Glucose controls glucagon secretion by directly modulating cAMP in alpha cells". Diabetologia. 62 (7): 1212–1224. doi:10.1007/s00125-019-4857-6. PMC 6560012. PMID 30953108.
- ^ Hue L, Rider MH (July 1987). "Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues". The Biochemical Journal. 245 (2): 313–24. doi:10.1042/bj2450313. PMC 1148124. PMID 2822019.
- ^ Claus TH, El-Maghrabi MR, Regen DM, Stewart HB, McGrane M, Kountz PD, Nyfeler F, Pilkis J, Pilkis SJ (1984). The Role of Fructose 2,6-Bisphosphate in the Regulation of Carbohydrate Metabolism. Current Topics in Cellular Regulation. Vol. 23. pp. 57–86. doi:10.1016/b978-0-12-152823-2.50006-4. ISBN 9780121528232. PMID 6327193.
- ^ Feliú JE, Hue L, Hers HG (August 1976). "Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes". Proceedings of the National Academy of Sciences of the United States of America. 73 (8): 2762–6. Bibcode:1976PNAS...73.2762F. doi:10.1073/pnas.73.8.2762. PMC 430732. PMID 183209.
- ^ Jiang G, Zhang BB (April 2003). "Glucagon and regulation of glucose metabolism". Am J Physiol Endocrinol Metab. 284 (4): E671-8. doi:10.1152/ajpendo.00492.2002. PMID 12626323.
- ^ Hayashi Y (January 2021). "Glucagon regulates lipolysis and fatty acid oxidation through inositol triphosphate receptor 1 in the liver". Diabetes Investig. 12 (1): 32–34. doi:10.1111/jdi.13315. PMC 7779274. PMID 32506830.
- ^ Swenson TL, Porter JW (Mar 25, 1985). "Mechanism of glucagon inhibition of liver acetyl-CoA carboxylase. Interrelationship of the effects of phosphorylation, polymer-protomer transition, and citrate on enzyme activity". The Journal of Biological Chemistry. 2460 (6): 3791–3797. doi:10.1016/S0021-9258(19)83693-1. PMID 2857722.
- ^ Wang Y, Yu W, Li S, Guo D, He J, Wang Y (March 11, 2022). "Acetyl-CoA Carboxylases and Diseases". Frontiers in Oncology. 12. doi:10.3389/fonc.2022.836058. PMC 8963101. PMID 35359351.
- ^ John AM, Schwartz RA (December 2016). "Glucagonoma syndrome: a review and update on treatment". Journal of the European Academy of Dermatology and Venereology. 30 (12): 2016–2022. doi:10.1111/jdv.13752. PMID 27422767. S2CID 1228654.
- ^ Oberg K (December 2010). "Pancreatic endocrine tumors". Seminars in Oncology. 37 (6): 594–618. doi:10.1053/j.seminoncol.2010.10.014. PMID 21167379.
- ^ Fasanmade OA, Odeniyi IA, Ogbera AO (June 2008). "Diabetic ketoacidosis: diagnosis and management". African Journal of Medicine and Medical Sciences. 37 (2): 99–105. PMID 18939392.
- ^ Kimball C, Murlin J (1923). "Aqueous extracts of pancreas III. Some precipitation reactions of insulin". J. Biol. Chem. 58 (1): 337–348. doi:10.1016/S0021-9258(18)85474-6.
- ^ Staub A, Sinn L, Behrens OK (June 1953). "Purification and crystallization of hyperglycemic glycogenolytic factor (HGF)". Science. 117 (3049): 628–9. Bibcode:1953Sci...117..628S. doi:10.1126/science.117.3049.628. PMID 13056638.
- ^ Bromer W, Winn L, Behrens O (1957). "The amino acid sequence of glucagon V. Location of amide groups, acid degradation studies and summary of sequential evidence". J. Am. Chem. Soc. 79 (11): 2807–2810. doi:10.1021/ja01568a038.
- ^ Lundqvist G, Edwards J, Wide L (January 1976). "A solid phase radioimmunoassay for pancreatic glucagon". Upsala Journal of Medical Sciences. 81 (2): 65–69. doi:10.3109/03009737609179024. PMID 785743.
- ^ a b c d e f Molteni M, Chen E (September 30, 2023). "GLP-1 drugs are transforming diabetes, obesity and more. Could a Nobel be next?". STAT News. Retrieved October 16, 2024.
External links
[edit]- PDBe-KB provides an overview of all the structure information available in the PDB for Human Glucagon

