Phases of ice
The phases of ice are all possible states of matter for water as a solid. Currently, 19 phases, including both crystalline and amorphous ice, have been observed at various densities.
Theory[edit]
Most liquids under increased pressure freeze at higher temperatures because the pressure helps to hold the molecules together. However, the strong hydrogen bonds in water make it different: for some pressures higher than 1 atm (0.10 MPa), water freezes at a temperature below 0 °C. Subjected to higher pressures and varying temperatures, ice can form in nineteen separate known crystalline phases. With care, at least fifteen of these phases (one of the known exceptions being ice X) can be recovered at ambient pressure and low temperature in metastable form.[1][2] The types are differentiated by their crystalline structure, proton ordering,[3] and density. There are also two metastable phases of ice under pressure, both fully hydrogen-disordered; these are Ice IV and Ice XII.
Crystal structure[edit]


The accepted crystal structure of ordinary ice was first proposed by Linus Pauling in 1935. The structure of ice Ih is the wurtzite lattice, roughly one of crinkled planes composed of tessellating hexagonal rings, with an oxygen atom on each vertex, and the edges of the rings formed by hydrogen bonds. The planes alternate in an ABAB pattern, with B planes being reflections of the A planes along the same axes as the planes themselves.[4] The distance between oxygen atoms along each bond is about 275 pm and is the same between any two bonded oxygen atoms in the lattice. The angle between bonds in the crystal lattice is very close to the tetrahedral angle of 109.5°, which is also quite close to the angle between hydrogen atoms in the water molecule (in the gas phase), which is 105°.
This tetrahedral bonding angle of the water molecule essentially accounts for the unusually low density of the crystal lattice – it is beneficial for the lattice to be arranged with tetrahedral angles even though there is an energy penalty in the increased volume of the crystal lattice. As a result, the large hexagonal rings leave almost enough room for another water molecule to exist inside. This gives naturally occurring ice its rare property of being less dense than its liquid form. The tetrahedral-angled hydrogen-bonded hexagonal rings are also the mechanism that causes liquid water to be densest at 4 °C. Close to 0 °C, tiny hexagonal ice Ih-like lattices form in liquid water, with greater frequency closer to 0 °C. This effect decreases the density of the water, causing it to be densest at 4 °C when the structures form infrequently.
In the most common form of ice, ice Ih, the crystal structure is characterized by the oxygen atoms forming hexagonal symmetry with near tetrahedral bonding angles. This structure is stable down to −268 °C (5 K; −450 °F), as evidenced by x-ray diffraction[5] and extremely high resolution thermal expansion measurements.[6] Ice Ih is also stable under applied pressures of up to about 210 megapascals (2,100 atm) where it transitions into ice III or ice II.[7]
Amorphous ice[edit]
While most forms of ice are crystalline, several amorphous (or "vitreous") forms of ice also exist. Such ice is an amorphous solid form of water, which lacks long-range order in its molecular arrangement. Amorphous ice is produced either by rapid cooling of liquid water to its glass transition temperature (about 136 K or −137 °C) in milliseconds (so the molecules do not have enough time to form a crystal lattice), or by compressing ordinary ice at low temperatures. The most common form on Earth, low-density ice, is usually formed in the laboratory by a slow accumulation of water vapor molecules (physical vapor deposition) onto a very smooth metal crystal surface under 120 K. In outer space it is expected to be formed in a similar manner on a variety of cold substrates, such as dust particles.[8] By contrast, hyperquenched glassy water (HGW) is formed by spraying a fine mist of water droplets into a liquid such as propane around 80 K, or by hyperquenching fine micrometer-sized droplets on a sample-holder kept at liquid nitrogen temperature, 77 K, in a vacuum. Cooling rates above 104 K/s are required to prevent crystallization of the droplets. At liquid nitrogen temperature, 77 K, HGW is kinetically stable and can be stored for many years.
Amorphous ices have the property of suppressing long-range density fluctuations and are, therefore, nearly hyperuniform.[9] Despite the epithet "ice", classification analysis utilizing neural networks has shown that amorphous ices are glasses.[10]
Pressure-dependent states[edit]

Ice from a theorized superionic water may possess two crystalline structures. At pressures in excess of 500,000 bars (7,300,000 psi) such superionic ice would take on a body-centered cubic structure. However, at pressures in excess of 1,000,000 bars (15,000,000 psi) the structure may shift to a more stable face-centered cubic lattice. Some estimates suggest that at an extremely high pressures of ~1.55 TPa, ice would develop metalic properties.[12] or 5.62 TPa.[13]
Heat and entropy[edit]
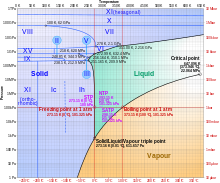
Ice, water, and water vapour can coexist at the triple point, which is exactly 273.16 K (0.01 °C) at a pressure of 611.657 Pa.[15][16] The kelvin was defined as 1/273.16 of the difference between this triple point and absolute zero,[17] though this definition changed in May 2019.[18] Unlike most other solids, ice is difficult to superheat. In an experiment, ice at −3 °C was superheated to about 17 °C for about 250 picoseconds.[19]

The latent heat of melting is 5987 J/mol, and its latent heat of sublimation is 50911 J/mol. The high latent heat of sublimation is principally indicative of the strength of the hydrogen bonds in the crystal lattice. The latent heat of melting is much smaller, partly because liquid water near 0 °C also contains a significant number of hydrogen bonds. By contrast, the structure of ice II is hydrogen-ordered, which helps to explain the entropy change of 3.22 J/mol when the crystal structure changes to that of ice I. Also, ice XI, an orthorhombic, hydrogen-ordered form of ice Ih, is considered the most stable form at low temperatures.
The transition entropy from ice XIV to ice XII is estimated to be 60% of Pauling entropy based on DSC measurements.[20] The formation of ice XIV from ice XII is more favoured at high pressure.[21]
When medium-density amorphous ice is compressed, released and then heated, it releases a large amount of heat energy, unlike other water ices which return to their normal form after getting similar treatment.[22]
Hydrogen disorder[edit]

The hydrogen atoms in the crystal lattice lie very nearly along the hydrogen bonds, and in such a way that each water molecule is preserved. This means that each oxygen atom in the lattice has two hydrogens adjacent to it: at about 101 pm along the 275 pm length of the bond for ice Ih. The crystal lattice allows a substantial amount of disorder in the positions of the hydrogen atoms frozen into the structure as it cools to absolute zero. As a result, the crystal structure contains some residual entropy inherent to the lattice and determined by the number of possible configurations of hydrogen positions that can be formed while still maintaining the requirement for each oxygen atom to have only two hydrogens in closest proximity, and each H-bond joining two oxygen atoms having only one hydrogen atom.[23] This residual entropy S0 is equal to 3.4±0.1 J mol−1 K−1 .[24]
Calculations[edit]
There are various ways of approximating this number from first principles. The following is the one used by Linus Pauling.[25][26]
Suppose there are a given number N of water molecules in an ice lattice. To compute its residual entropy, we need to count the number of configurations that the lattice can assume. The oxygen atoms are fixed at the lattice points, but the hydrogen atoms are located on the lattice edges. The problem is to pick one end of each lattice edge for the hydrogen to bond to, in a way that still makes sure each oxygen atom is bond to two hydrogen atoms.
The oxygen atoms can be divided into two sets in a checkerboard pattern, shown in the picture as black and white balls. Focus attention on the oxygen atoms in one set: there are N/2 of them. Each has four hydrogen bonds, with two hydrogens close to it and two far away. This means there are allowed configurations of hydrogens for this oxygen atom (see Binomial coefficient). Thus, there are 6N/2 configurations that satisfy these N/2 atoms. But now, consider the remaining N/2 oxygen atoms: in general they won't be satisfied (i.e., they will not have precisely two hydrogen atoms near them). For each of those, there are 24 = 16 possible placements of the hydrogen atoms along their hydrogen bonds, of which 6 are allowed. So, naively, we would expect the total number of configurations to be
Using Boltzmann's entropy formula, we conclude that
The same answer can be found in another way. First orient each water molecule randomly in each of the 6 possible configurations, then check that each lattice edge contains exactly one hydrogen atom. Assuming that the lattice edges are independent, then the probability that a single edge contains exactly one hydrogen atom is 1/2, and since there are 2N edges in total, we obtain a total configuration count , as before.
Refinements[edit]

This estimate is 'naive', as it assumes the six out of 16 hydrogen configurations for oxygen atoms in the second set can be independently chosen, which is false. More complex methods can be employed to better approximate the exact number of possible configurations, and achieve results closer to measured values. Nagle (1966) used a series summation to obtain .[27]
As an illustrative example of refinement, consider the following way to refine the second estimation method given above. According to it, six water molecules in a hexagonal ring would allow configurations. However, by explicit enumeration, there are actually 730 configurations. Now in the lattice, each oxygen atom participates in 12 hexagonal rings, so there are 2N rings in total for N oxygen atoms, or 2 rings for each oxygen atom, giving a refined result of .[28]
Known phases[edit]
These phases are named according to the Bridgman nomenclature. The majority have only been created in the laboratory at different temperatures and pressures.[29]
| Phase | Year of discovery | Temperature thresholds | Pressure thresholds | Density | Crystal form | Other characteristics |
|---|---|---|---|---|---|---|
| Ice Ih | NA (always known) | 0 °C (32 °F) (freezing) | NA (atmospheric) | 0.917 g/cm3 | Hexagonal | Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic. Has a refractive index of 1.31. |
| Ice Ic | 1943 | 130 and 220 K (−143 and −53 °C) (formation)/240 K (−33 °C) (conversion to Ice Ih)[30][31] | NA (atmospheric) | Similar to Ice Ih | Diamond[32] | A metastable cubic crystalline variant of ice. |
| Low-density amorphous ice (LDA) | NA (atmospheric or lower) | 0.94 g/cm3 [33] | NA (amorphous) | More viscous than normal water.[33][34][35] | ||
| Medium-density amorphous ice (MDA) | 2023[22][36] | −200 °C (−328.0 °F) (freezing) | NA (requires shear force) | 1.06±0.06 g cm3[37] | NA (amorphous) | Experimental procedure generates shear force by crushing ice into powder with centimeter-wide stainless-steel balls added to its container. |
| High-density amorphous ice (HDA) | 1984[38] | <140 K (−133 °C) (normal formation); <30 K (−243.2 °C) (vapor deposition)[33][39] 77 K (−196.2 °C) (stability point)[38] | At 77 K (−196.2 °C): 1.6 GPa (formation from Ih);[38] 0.5nbsp;GPa (formation from LDA)[40] | 1.17 g/cm3 (ambient pressure)[38] | NA (amorphous) | |
| Very high-density amorphous ice (VHDA) | 1996[41] | 160 K (−113 °C) (formation from HDA); 77 K (−196.2 °C) (stability point) | 1 and 2 GPa (formation at 160 K (−113 °C)); ambient (at 77 K (−196.2 °C)) | 1.26 g/cm3 (77 K (−196.2 °C); ambient pressure)[42] | NA (amorphous) | |
| Ice II | 1900[43] | 190 K (−83 °C)-210 K (−63 °C) (formation from ice Ih); 77 K (−196.2 °C) (stability point)[43] | 300 MPa[44] | Rhombohedral | ||
| Ice III | 1900[43] | 250 K (−23 °C) (formation from liquid water); 77 K (−196.2 °C) (stability point)[43] | 300 MPa (formation from liquid water)[44] | 1160 kg/m3 (at 350 MPa)[45] | Tetragonal | Very high relative permittivity at 117. A specific gravity of 1.16 with respect to water. |
| Ice IV | 1900[43] | 190 K (−83 °C)-210 K (−63 °C) (formation from HDA); 77 K (−196.2 °C) (stability point) | 810 MPa (formation from HDA) | Rhombohedral | Typically requires a nucleating agent to form.[46] | |
| Ice V | 253 K (−20 °C) (formation from liquid water) | 500 MPa (formation from liquid water) [47] | 1.24 g cm3 (at 350 MPa).[48] | Monoclinic | Most complicated structure of all the phases. Includes 4-membered, 5-membered, 6-membered, and 8-membered rings and a total of 28 molecules in the unit cell.[49][50] | |
| Ice VI | 1912[51] | 270 K (−3 °C) (formation from liquid water); 130 K (−143 °C) - 355 K (82 °C) (stability range) | 1.1 GPa (formation from liquid water) [47] | 1.31 g/cm3[52] | Tetragonal | Exhibits Debye relaxation.[53] |
| Ice VII | 270 K (−3 °C) (formation from ice Ih) | 1.1 GPa (formation from ice Ih); 5 GPa (formation of tetragonal structure)[54] | Cubic/tetragonal | The hydrogen atoms' positions are disordered. Exhibits Debye relaxation. The hydrogen bonds form two interpenetrating lattices. Tetragonal form known as Ice VIIt. | ||
| Ice VIII | <5 °C (278 K) (formation from ice VII) | 2.1 GPa (formation from ice VII) | Cubic | Hydrogen atoms assume fixed positions.[55] | ||
| Ice IX | 165 K (−108 °C) (formation from ice III); <140 K (−133 °C) (stability point) | 200 MPa-400 MPa (stability range) | 1.16 g/cm3 | Tetragonal | Proton-ordered equivalent to Ice III.[56] | |
| Ice X | 165 K (−108 °C) (formation from ice III); <140 K (−133 °C) (stability point) | 30-70 GPa (from ice VII)[57][54] | Cubic | Has symmetrized hydrogen bonds - a hydrogen atom is found at the center of two oxygen atoms. | ||
| Ice XI | 165 K (−108 °C) (formation from ice III); <140 K (−133 °C) (stability point) | 30-70 GPa (from ice VII)[57][54] | Orthorhombic | Ferroelectric. The most stable configuration of ice Ih.[58] | ||
| Ice XII | 1996[59] | 260 K (−13 °C; 8 °F) (formation from liquid water); 77 K (−196.2 °C; −321.1 °F) (formation from ice Ih); 183 K (−90 °C) (formation from HDA ice) | 0.55 gigapascals (5,400 atm) (formation from liquid water); 0.81-1.00 GPa/min (from ice Ih); 810 MPa (formation from HDA ice) | 1.3 g·cm−3 (at 127 K (−146 °C)) | Tetragonal | Metastable. Observed in the phase space of ice V and ice VI. A topological mix of seven- and eight-membered rings, a 4-connected net (4-coordinate sphere packing)—the densest possible arrangement without hydrogen bond interpenetration. |
| Ice XIII | 2006[60] | 130 K (−143 °C) (formation from liquid water) [61] | 500 MPa (formation from liquid water)[61] | Monoclinic | The proton-ordered form of ice V.[61] | |
| Ice XIV | 2006[60] | <118 K (−155 °C) (formation from ice XII); <140 K (−133 °C) (stability point) | 1.2GPa (formation from ice XII)[61] | Orthorhombic | The proton-ordered form of ice XII.[61] Formation requires HCL.[62] | |
| Ice XV | 2009[63] | 80 K (−193.2 °C)- 108 kelvins (−165 degrees Celsius) (formation from liquid water) | 1.1GPa (formation from liquid water) | A proton-ordered form of ice VI formed by cooling water to around 80–108 K at 1.1 GPa. | ||
| Ice XVI | 2016[64][65][66][67][68] | <118 K (−155 °C) (formation from ice III); <140 K (−133 °C) (stability point) | 1.2GPa (from ice VII)[61] | 0.81 g/cm3)[69] | The least dense crystalline form of water, topologically equivalent to the empty structure of sII clathrate hydrates. Transforms into the stacking-faulty ice Ic and further into ordinary ice Ih when above 145–147 K at positive pressures. Theoretical studies predict ice XVI to be thermodynamically stable at negative pressures (that is under tension).[11][70] | |
| Square ice | 2014[71] | Room temperature (in the presence of graphene) | 10GPa [72] | Square | Formation likely driven by the van der Waals force, which allows water vapor and liquid water to pass through laminated sheets of graphene oxide, unlike smaller molecules such as helium.[72] | |
| Ice XVII | <118 K (−155 °C) (formation from ice III); <140 K (−133 °C) (stability point) | 1.2GPa (from ice III) | Near that of ice XVI.[73][74][67] | Hexagonal | A porous crystalline phase with helical channels, with density Formed by placing hydrogen-filled ice in a vacuum and increasing the temperature until the hydrogen molecules escape.[73] | |
| Ice XVIII | <118 K (−155 °C) (formation from ice III); <140 K (−133 °C) (stability point) | 1.2GPa (from ice VII)[61] | A form of water also known as superionic water or superionic ice in which oxygen ions develop a crystalline structure while hydrogen ions move freely. | |||
| Ice XIX | 2018[75] | <100 K (−173 °C) (formation from ice VIh); [76] | 2GPa (formation from ice VIh)[76] | Formation involves HCl.[75][76][77] |
History of research[edit]

Ice II[edit]
The properties of ice II were first described and recorded by Gustav Heinrich Johann Apollon Tammann in 1900 during his experiments with ice under high pressure and low temperatures. Having produced ice III, Tammann then tried condensing the ice at a temperature between −70 and −80 °C (203 and 193 K; −94 and −112 °F) under 200 MPa (2,000 atm) of pressure. Tammann noted that in this state ice II was denser than he had observed ice III to be. He also found that both types of ice can be kept at normal atmospheric pressure in a stable condition so long as the temperature is kept at that of liquid air, which slows the change in conformation back to ice Ih.[43]
In later experiments by Bridgman in 1912, it was shown that the difference in volume between ice II and ice III was in the range of 0.0001 m3/kg (2.8 cu in/lb). This difference hadn't been discovered by Tammann due to the small change and was why he had been unable to determine an equilibrium curve between the two. The curve showed that the structural change from ice III to ice II was more likely to happen if the medium had previously been in the structural conformation of ice II. However, if a sample of ice III that had never been in the ice II state was obtained, it could be supercooled even below −70 °C without it changing into ice II. Conversely, however, any superheating of ice II was not possible in regards to retaining the same form. Bridgman found that the equilibrium curve between ice II and ice IV was much the same as with ice III, having the same stability properties and small volume change. The curve between ice II and ice V was extremely different, however, with the curve's bubble being essentially a straight line and the volume difference being almost always 0.0000545 m3/kg (1.51 cu in/lb).[43]
Search for a hydrogen-disordered counterpart[edit]
As ice II is completely hydrogen ordered, the presence of its disordered counterpart is a great matter of interest. Shephard et al.[78] investigated the phase boundaries of NH4F-doped ices because NH4F has been reported to be a hydrogen disordering reagent. However, adding 2.5 mol% of NH4F resulted in the disappearance of ice II instead of the formation of a disordered ice II. According to the DFC calculation by Nakamura et al.,[79] the phase boundary between ice II and its disordered counterpart is estimated to be in the stability region of liquid water.
Ice IV[edit]
1981 research by Engelhardt and Kamb elucidated crystal structure of ice IV through a low-temperature single-crystal X-ray diffraction, describing it as a rhombohedral unit cell with a space group of R-3c.[80] This research mentioned that the structure of ice IV could be derived from the structure of ice Ic by cutting and forming some hydrogen bondings and adding subtle structural distortions. Shephard et al.[81] compressed the ambient phase of NH4F, an isostructural material of ice, to obtain NH4F II, whose hydrogen-bonded network is similar to ice IV. As the compression of ice Ih results in the formation of high-density amorphous ice (HDA), not ice IV, they claimed that the compression-induced conversion of ice I into ice IV is important, naming it "Engelhardt–Kamb collapse" (EKC). They suggested that the reason why we cannot obtain ice IV directly from ice Ih is that ice Ih is hydrogen-disordered; if oxygen atoms are arranged in the ice IV structure, hydrogen bonding may not be formed due to the donor-acceptor mismatch.[82] and Raman [83]
The disordered nature of Ice IV was confirmed by neutron powder diffraction studies by Lobban (1998) [84] and Klotz et al. (2003).[85] In addition, the entropy difference between ice VI (disordered phase) and ice IV is very small, according to Bridgman's measurement.[86]
Several organic nucleating reagents had been proposed to selectively crystallize ice IV from liquid water,[87] but even with such reagents, the crystallization of ice IV from liquid water was very difficult and seemed to be a random event. In 2001, Salzmann and his coworkers reported a whole new method to prepare ice IV reproducibly;[88] when high-density amorphous ice (HDA) is heated at a rate of 0.4 K/min and a pressure of 0.81 GPa, ice IV is crystallized at about 165 K. What governs the crystallization products is the heating rate; fast heating (over 10 K/min) results in the formation of single-phase ice XII.
Search for a hydrogen-ordered counterpart[edit]
The ordered counterpart of Ice IV has never been reported yet. 2011 research by Salzmann's group reported more detailed DSC data where the endothermic feature becomes larger as the sample is quench-recovered at higher pressure. They proposed three scenarios to explain the experimental results: weak hydrogen-ordering, orientational glass transition, and mechanical distortions.[89] reported the DSC thermograms of HCl-doped ice IV finding an endothermic feature at about 120 K. Ten years later, Rosu-Finsen and Salzmann (2021) [90]
Ice XI[edit]


Ice XI is the hydrogen-ordered form of the ordinary form of ice. The total internal energy of ice XI is about one sixth lower than ice Ih, so in principle it should naturally form when ice Ih is cooled to below 72 K. The low temperature required to achieve this transition is correlated with the relatively low energy difference between the two structures.[91] Hints of hydrogen-ordering in ice had been observed as early as 1964, when Dengel et al. attributed a peak in thermo-stimulated depolarization (TSD) current to the existence of a proton-ordered ferroelectric phase.[92] However, they could not conclusively prove that a phase transition had taken place, and Onsager pointed out that the peak could also arise from the movement of defects and lattice imperfections. Onsager suggested that experimentalists look for a dramatic change in heat capacity by performing a careful calorimetric experiment. A phase transition to ice XI was first identified experimentally in 1972 by Shuji Kawada and others.[93][94][95]
Water molecules in ice Ih are surrounded by four semi-randomly directed hydrogen bonds. Such arrangements should change to the more ordered arrangement of hydrogen bonds found in ice XI at low temperatures, so long as localized proton hopping is sufficiently enabled; a process that becomes easier with increasing pressure.[96] Correspondingly, ice XI is believed to have a triple point with hexagonal ice and gaseous water at (~72 K, ~0 Pa). Ice Ih that has been transformed to ice XI and then back to ice Ih, on raising the temperature, retains some hydrogen-ordered domains and more easily transforms back to ice XI again.[97] A neutron powder diffraction study found that small hydrogen-ordered domains can exist up to 111 K.[98]
There are distinct differences in the Raman spectra between ices Ih and XI, with ice XI showing much stronger peaks in the translational (~230 cm−1), librational (~630 cm−1) and in-phase asymmetric stretch (~3200 cm−1) regions.[99][100]
Ice Ic also has a proton-ordered form. The total internal energy of ice XIc was predicted as similar as ice XIh [101]
Ferroelectric properties[edit]
Ice XI is ferroelectric, meaning that it has an intrinsic polarization. To qualify as a ferroelectric it must also exhibit polarization switching under an electric field, which has not been conclusively demonstrated but which is implicitly assumed to be possible.[102] Cubic ice also has a ferrolectric phase and in this case the ferroelectric properties of the ice have been experimentally demonstrated on monolayer thin films.[103] In a similar experiment, ferroelectric layers of hexagonal ice were grown on a platinum (111) surface. The material had a polarization that had a decay length of 30 monolayers suggesting that thin layers of ice XI can be grown on substrates at low temperature without the use of dopants.[104] One-dimensional nano-confined ferroelectric ice XI was created in 2010.[105]
Ice XV[edit]
Although the parent phase ice VI was discovered in 1935, corresponding proton-ordered forms (ice XV) had not been observed until 2009. Theoretically, the proton ordering in ice VI was predicted several times; for example, density functional theory calculations predicted the phase transition temperature is 108 K and the most stable ordered structure is antiferroelectric in the space group Cc, while an antiferroelectric P212121 structure were found 4 K per water molecule higher in energy.[106]
On 14 June 2009, Christoph Salzmann and colleagues at the University of Oxford reported having experimentally reported an ordered phase of ice VI, named ice XV, and say that its properties differ significantly from those predicted. In particular, ice XV is antiferroelectric rather than ferroelectric as had been predicted.[107][108]
Ice XVII[edit]

In 2016, the discovery of a new form of ice was announced.[65] Characterized as a "porous water ice metastable at atmospheric temperatures", this new form was discovered by taking a filled ice and removing the non-water components, leaving the crystal structure behind, similar to how ice XVI, another porous form of ice, was synthesized from a clathrate hydrate.[64][65][66][67][68]
To create ice XVII, the researchers first produced filled ice in a stable phase named C0 from a mixture of hydrogen (H2) and water (H2O), using temperatures from 100 to 270 K (−173 to −3 °C; −280 to 26 °F) and pressures from 360 to 700 MPa (52,000 to 102,000 psi; 3,600 to 6,900 atm).[65][a] The filled ice is then placed in a vacuum, and the temperature gradually increased until the hydrogen frees itself from the crystal structure.[65][66][b] The resulting form is metastable at room pressure while under 120 K (−153 °C; −244 °F), but collapses into ice Ih (ordinary ice) when brought above 130 K (−143 °C; −226 °F).[65][66] The crystal structure is hexagonal in nature, and the pores are helical channels with a diameter of about 6.10 Å (6.10×10−10 m; 2.40×10−8 in).[65][66]
Cubic ice[edit]
It was reported in 2020 that cubic ice based on heavy water (D2O) can be formed from ice XVII.[109] This was done by heating specially prepared D2O ice XVII powder.[109] The result was free of structural deformities compared to standard cubic ice, or ice Isd.[109][110] This discovery was reported around the same time another research group announced that they were able to obtain pure D2O cubic ice by first synthesizing filled ice in the C2 phase, and then decompressing it.[111][65][a]
Ice XVIII (superionic water)[edit]
In 1988, predictions of the so-called superionic water state were made.[112] In superionic water, water molecules break apart and the oxygen ions crystallize into an evenly spaced lattice while the hydrogen ions float around freely within the oxygen lattice.[113] The freely mobile hydrogen ions make superionic water almost as conductive as typical metals, making it a superionic conductor.[114] It is distinct from ionic water, which is a hypothetical liquid state characterized by a disordered soup of hydrogen and oxygen ions.
The initial evidence came from optical measurements of laser-heated water in a diamond anvil cell,[115] and from optical measurements of water shocked by extremely powerful lasers.[116] The first definitive evidence for the crystal structure of the oxygen lattice in superionic water came from x-ray measurements on laser-shocked water which were reported in 2019.[114] In 2005 Laurence Fried led a team at Lawrence Livermore National Laboratory to recreate the formative conditions of superionic water. Using a technique involving smashing water molecules between diamonds and super heating it with lasers they observed frequency shifts which indicated that a phase transition had taken place. The team also created computer models which indicated that they had indeed created superionic water.[117] In 2013 Hugh F. Wilson, Michael L. Wong, and Burkhard Militzer at the University of California, Berkeley published a paper predicting the face-centered cubic lattice structure that would emerge at higher pressures.[118] Additional experimental evidence was found by Marius Millot and colleagues in 2018 by inducing high pressure on water between diamonds and then shocking the water using a laser pulse.[116][119]
As of 2013[update], it is theorized that superionic ice can possess two crystalline structures. At pressures in excess of 50 GPa (7,300,000 psi) it is predicted that superionic ice would take on a body-centered cubic structure. However, at pressures in excess of 100 GPa, and temperatures above 2000 K, it is predicted that the structure would shift to a more stable face-centered cubic lattice.[118] The ice appears black in color.[116][119]
In 2018, researchers at LLNL squeezed water between two pieces of diamond with a pressure of 2,500 MPa (360,000 psi). The water was squeezed into Ice VII, which is 60 percent denser than normal water.[120] The compressed ice was then transported to the University of Rochester where it was blasted by a pulse of laser light. The reaction created conditions like those inside of ice giants such as Uranus and Neptune by heating up the ice thousands of degrees under a pressure a million times greater than the Earth's atmosphere in only 10 to 20 billionths of a second. The experiment concluded that the current in the conductive water was indeed carried by ions rather than electrons and thus pointed to the water being superionic.[120] More recent experiments from the same Lawrence Livermore National Laboratory team used x-ray crystallography on laser-shocked water droplets to determine that the oxygen ions enter a face-centered-cubic phase, which was dubbed ice XVIII and reported in the journal Nature in May 2019.[114]
Ice XIX[edit]
The first report regarding ice XIX was published in 2018 by Thomas Loerting's group from Austria.[75] They quenched HCl-doped ice VI to 77 K at different pressures between 1.0 and 1.8 GPa to collect differential scanning calorimetry (DSC) thermograms, dielectric spectrum, Raman spectrum, and X-ray diffraction patterns. In the DSC signals, there was an endothermic feature at about 110 K in addition to the endotherm corresponding to the ice XV-VI transition. Additionally, the Raman spectra, dielectric properties, and the ratio of the lattice parameters differed from those of ice XV. Based on these observations, they proposed the existence of a second hydrogen-ordered phase of ice VI, naming it ice beta-XV.
In 2019, Alexander Rosu-Finsen and Christoph Salzman argued that there was no need to consider this to be a new phase of ice, and proposed a "deep-glassy" state scenario.[121] According to their DSC data, the size of the endothermic feature depends not only on quench-recovery pressure but also on the heating rate and annealing duration at 93 K. They also collected neutron diffraction profiles of quench-recovered deuterium chloride-doped, D2O ice VI/XV prepared at different pressures of 1.0, 1.4 and 1.8 GPa, to show that there were no significant differences among them. They concluded that the low-temperature endotherm originated from kinetic features related to glass transitions of deep glassy states of disordered ice VI.
Distinguishing between the two scenarios (new hydrogen-ordered phase vs. deep-glassy disordered ice VI) became an open question and the debate between the two groups has continued. Thoeny et al. (Loerting's group) [122] collected another series of Raman spectra of ice beta-XV, and reported that (i) ice XV prepared by the protocol reported previously contains both ice XV and ice beta-XV domains; (ii) upon heating, Raman spectra of ice beta-XV showed loss of H-order. In contrast, Salzmann's group again argued for the plausibility of a 'deep-glassy state' scenario based on neutron diffraction and neutron inelastic scattering experiments.[123] Based on their experimental results, ice VI and deep-glassy ice VI share very similar features based on both elastic (diffraction) scattering and inelastic scattering experiments, and are different from the properties of ice XV.
In 2021, further crystallographic evidence for a new phase (ice XIX) was individually reported by three groups: Yamane et al. (Hiroyuki Kagi and Kazuki Komatsu's group from Japan), Gasser et al. (Loerting's group), and Salzmann's group. Yamane et al. [77] collected neutron diffraction profiles in situ (i.e. under high pressure) and found new Bragg features completely different from both ice VI and ice XV. They performed Rietveld refinement of the profiles based on the supercell of ice XV and proposed some leading candidates for the space group of ice XIX: P-4, Pca21, Pcc2, P21/a, and P21/c. They also measured dielectric spectra in situ and determined phase boundaries of ices VI/XV/XIX. They found that the sign of the slope of the boundary turns negative from positive at 1.6 GPa indicating the existence of two different phases by the Clausius–Clapeyron relation.
Gasser et al. [124] also collected powder neutron diffractograms of quench-recovered ices VI, XV, and XIX and found similar crystallographic features to those reported by Yamane et al., concluding that P-4 and Pcc2 are the plausible space group candidates. Both Yamane et al.'s and Gasser et al.'s results suggested a partially hydrogen-ordered structure. Gasser et al. also found an isotope effect using DSC; the low-temperature endotherm for DCl-doped D2O ice XIX was significantly smaller than that of HCl-doped H2O ice XIX, and that doping of 0.5% of H2O into D2O is sufficient for the ordering transition.
Several months later, Salzmann et al. published a paper based on in-situ powder neutron diffraction experiments of ice XIX.[125] In a change from their previous reports, they accepted the idea of the new phase (ice XIX) as they observed similar features to the previous two reports. However, they refined their diffraction profiles based on a disordered structural model (Pbcn) and argued that new Bragg reflections can be explained by distortions of ice VI, so ice XIX may still be regarded as a deep-glassy state of ice VI. The crystal structure of ice XIX including hydrogen order/disorder is still under debate as of 2022.
Practical implications[edit]
Earth's natural environment[edit]


Virtually all ice in the biosphere is ice Ih (pronounced: ice one h, also known as ice-phase-one). Ice Ih exhibits many peculiar properties that are relevant to the existence of life and regulation of global climate. [126] For instance, its density is lower than that of liquid water. This is attributed to the presence of hydrogen bonds which causes atoms to become closer in the liquid phase.[127] Because of this, ice Ih floats on water, which is highly unusual when compared to other materials. The solid phase of materials is usually more closely and neatly packed and has a higher density than the liquid phase. When lakes freeze, they do so only at the surface while the bottom of the lake remains near 4 °C (277 K; 39 °F) because water is densest at this temperature. No matter how cold the surface becomes, there is always a layer at the bottom of the lake that is 4 °C (277 K; 39 °F). This anomalous behavior of water and ice is what allows fish to survive harsh winters. The density of ice Ih increases when cooled, down to about −211 °C (62 K; −348 °F); below that temperature, the ice expands again (negative thermal expansion).[5][6]
Besides ice Ih, a small amount of ice Ic may occasionally present in the upper atmosphere clouds.[128] It is believed to be responsible for the observation of Scheiner's halo, a rare ring that occurs near 28 degrees from the Sun or the Moon.[129] However, many atmospheric samples which were previously described as cubic ice were later shown to be stacking disordered ice with trigonal symmetry,[130][131][132] and it has been dubbed the ″most faceted ice phase in a literal and a more general sense.″[133] The first true samples of cubic ice were only reported in 2020.[134][135][136]
Low-density ASW (LDA), also known as hyperquenched glassy water, may be responsible for noctilucent clouds on Earth and is usually formed by deposition of water vapor in cold or vacuum conditions. Ice clouds form at and below the Earth's high latitude mesopause (~90 km) where temperatures have been observed to fall as to below 100 K.[137] It has been suggested that homogeneous nucleation of ice particles results in low density amorphous ice.[138] Amorphous ice is likely confined to the coldest parts of the clouds and stacking disordered ice I is thought to dominate elsewhere in these polar mesospheric clouds.[139]
In 2018, ice VII was identified among inclusions found in natural diamonds.[140] Due to this demonstration that ice VII exists in nature, the International Mineralogical Association duly classified ice VII as a distinct mineral.[141] The ice VII was presumably formed when water trapped inside the diamonds retained the high pressure of the deep mantle due to the strength and rigidity of the diamond lattice, but cooled down to surface temperatures, producing the required environment of high pressure without high temperature.[142]
Ice XI is thought to be a more stable conformation than ice Ih, and so it may form on Earth. However, the transformation is very slow. According to one report, in Antarctic conditions it is estimated to take at least 100,000 years to form without the assistance of catalysts.[citation needed] Ice XI was sought and found in Antarctic ice that was about 100 years old in 1998.[143] A further study in 2004 was not able to reproduce this finding, however, after studying Antarctic ice which was around 3000 years old.[144] The 1998 Antarctic study also claimed that the transformation temperature (ice XI => ice Ih) is −36 °C (237 K), which is far higher than the temperature of the expected triple point mentioned above (72 K, ~0 Pa). Ice XI was also found in experiments using pure water at very low temperature (~10 K) and low pressure – conditions thought to be present in the upper atmosphere.[145] Recently, small domains of ice XI were found to form in pure water; its phase transition back to ice Ih occurred at 72 K while under hydrostatic pressure conditions of up to 70 MPa.[146]
Human industry[edit]
Amorphous ice is used in some scientific experiments, especially in cryo-electron microscopy of biomolecules.[147] The individual molecules can be preserved for imaging in a state close to what they are in liquid water.
Ice XVII can repeatedly adsorb and release hydrogen molecules without degrading its structure.[65] The total amount of hydrogen that ice XVII can adsorb depends on the amount of pressure applied, but hydrogen molecules can be adsorbed by ice XVII even at pressures as low as a few millibars[c] if the temperature is under 40 K (−233.2 °C; −387.7 °F).[65][148] The adsorbed hydrogen molecules can then be released, or desorbed, through the application of heat.[148] This was an unexpected property of ice XVII, and could allow it to be used for hydrogen storage, an issue often mentioned in environmental technology.[65][148]
Aside from storing hydrogen via compression or liquification, it can also be stored within a solid substance, either via a reversible chemical process (chemisorption) or by having the hydrogen molecules attach to the substance via the van der Waals force (physisorption).[148] The storage method used by ice XVII falls in the latter category, physisorption.[148] In physisorption, there is no chemical reaction, and the chemical bond between the two atoms within a hydrogen molecule remains intact. Because of this, the number of adsorption–desorption cycles ice XVII can withstand is "theoretically infinite".[65][148]
One significant advantage of using ice XVII as a hydrogen storage medium is the low cost of the only two chemicals involved: hydrogen and water.[148] In addition, ice XVII has shown the ability to store hydrogen at an H2 to H2O molar ratio above 40%, higher than the theoretical maximum ratio for sII clathrate hydrates, another potential storage medium.[65] However, if ice XVII is used as a storage medium, it must be kept under a temperature of 130 K (−143 °C; −226 °F) or risk being destabilized.[148]
Outer space[edit]
In outer space, hexagonal crystalline ice (the predominant form found on Earth) is extremely rare. Known examples are typically associated with volcanic action.[149] Water in the interstellar medium is instead dominated by amorphous ice, making it likely the most common form of water in the universe.[150]
Amorphous ice can be separated from crystalline ice based on its near-infrared and infrared spectrum. At near-IR wavelengths, the characteristics of the 1.65, 3.1, and 4.53 μm water absorption lines are dependent on the ice temperature and crystal order.[151] The peak strength of the 1.65 μm band as well as the structure of the 3.1 μm band are particularly useful in identifying the crystallinity of water ice.[152][153]
At longer IR wavelengths, amorphous and crystalline ice have characteristically different absorption bands at 44 and 62 μm in that the crystalline ice has significant absorption at 62 μm while amorphous ice does not.[154] In addition, these bands can be used as a temperature indicator at very low temperatures where other indicators (such as the 3.1 and 12 μm bands) fail.[155] This is useful studying ice in the interstellar medium and circumstellar disks. However, observing these features is difficult because the atmosphere is opaque at these wavelengths, requiring the use of space-based infrared observatories.
Properties of the amorphous ice in the Solar System[edit]
In general, amorphous ice can form below ~130 K.[156] At this temperature, water molecules are unable to form the crystalline structure commonly found on Earth. Amorphous ice may also form in the coldest region of the Earth's atmosphere, the summer polar mesosphere, where noctilucent clouds exist.[157] These low temperatures are readily achieved in astrophysical environments such as molecular clouds, circumstellar disks, and the surfaces of objects in the outer Solar System. In the laboratory, amorphous ice transforms into crystalline ice if it is heated above 130 K, although the exact temperature of this conversion is dependent on the environment and ice growth conditions.[158] The reaction is irreversible and exothermic, releasing 1.26–1.6 kJ/mol.[158]
An additional factor in determining the structure of water ice is deposition rate. Even if it is cold enough to form amorphous ice, crystalline ice will form if the flux of water vapor onto the substrate is less than a temperature-dependent critical flux.[159] This effect is important to consider in astrophysical environments where the water flux can be low. Conversely, amorphous ice can be formed at temperatures higher than expected if the water flux is high, such as flash-freezing events associated with cryovolcanism.
At temperatures less than 77 K, irradiation from ultraviolet photons as well as high-energy electrons and ions can damage the structure of crystalline ice, transforming it into amorphous ice.[160][154] Amorphous ice does not appear to be significantly affected by radiation at temperatures less than 110 K, though some experiments suggest that radiation might lower the temperature at which amorphous ice begins to crystallize.[154]
Peter Jenniskens and David F. Blake demonstrated in 1994 that a form of high-density amorphous ice is also created during vapor deposition of water on low-temperature (< 30 K) surfaces such as interstellar grains. The water molecules do not fully align to create the open cage structure of low-density amorphous ice. Many water molecules end up at interstitial positions. When warmed above 30 K, the structure re-aligns and transforms into the low-density form.[33][39]
Molecular clouds, circumstellar disks, and the primordial solar nebula[edit]
Molecular clouds have extremely low temperatures (~10 K), falling well within the amorphous ice regime. The presence of amorphous ice in molecular clouds has been observationally confirmed.[161] When molecular clouds collapse to form stars, the temperature of the resulting circumstellar disk isn't expected to rise above 120 K, indicating that the majority of the ice should remain in an amorphous state.[159] However, if the temperature rises high enough to sublimate the ice, then it can re-condense into a crystalline form since the water flux rate is so low. This is expected to be the case in the circumstellar disk of IRAS 09371+1212, where signatures of crystallized ice were observed despite a low temperature of 30–70 K.[162]
For the primordial solar nebula, there is much uncertainty as to the crystallinity of water ice during the circumstellar disk and planet formation phases. If the original amorphous ice survived the molecular cloud collapse, then it should have been preserved at heliocentric distances beyond Saturn's orbit (~12 AU).[159]
Comets[edit]
The possibility of the presence of amorphous water ice in comets and the release of energy during the phase transition to a crystalline state was first proposed as a mechanism for comet outbursts.[163] Evidence of amorphous ice in comets is found in the high levels of activity observed in long-period, Centaur, and Jupiter Family comets at heliocentric distances beyond ~6 AU.[164] These objects are too cold for the sublimation of water ice, which drives comet activity closer to the Sun, to have much of an effect. Thermodynamic models show that the surface temperatures of those comets are near the amorphous/crystalline ice transition temperature of ~130 K, supporting this as a likely source of the activity.[165] The runaway crystallization of amorphous ice can produce the energy needed to power outbursts such as those observed for Centaur Comet 29P/Schwassmann–Wachmann 1.[166][167]
Kuiper Belt objects[edit]
With radiation equilibrium temperatures of 40–50 K,[168] the objects in the Kuiper Belt are expected to have amorphous water ice. While water ice has been observed on several objects,[169][170] the extreme faintness of these objects makes it difficult to determine the structure of the ices. The signatures of crystalline water ice was observed on 50000 Quaoar, perhaps due to resurfacing events such as impacts or cryovolcanism.[171]
Icy moons[edit]
The Near-Infrared Mapping Spectrometer (NIMS) on NASA's Galileo spacecraft spectroscopically mapped the surface ice of the Jovian satellites Europa, Ganymede, and Callisto. The temperatures of these moons range from 90 to 160 K,[172] warm enough that amorphous ice is expected to crystallize on relatively short timescales. However, it was found that Europa has primarily amorphous ice, Ganymede has both amorphous and crystalline ice, and Callisto is primarily crystalline.[173] This is thought to be the result of competing forces: the thermal crystallization of amorphous ice versus the conversion of crystalline to amorphous ice by the flux of charged particles from Jupiter. Closer to Jupiter than the other three moons, Europa receives the highest level of radiation and thus through irradiation has the most amorphous ice. Callisto is the farthest from Jupiter, receiving the lowest radiation flux and therefore maintaining its crystalline ice. Ganymede, which lies between the two, exhibits amorphous ice at high latitudes and crystalline ice at the lower latitudes. This is thought to be the result of the moon's intrinsic magnetic field, which would funnel the charged particles to higher latitudes and protect the lower latitudes from irradiation.[173] Ganymede's interior probably includes a liquid water ocean with tens to hundreds of kilometers of ice V at its base.[174]
The surface ice of Saturn's moon Enceladus was mapped by the Visual and Infrared Mapping Spectrometer (VIMS) on the NASA/ESA/ASI Cassini space probe. The probe found both crystalline and amorphous ice, with a higher degree of crystallinity at the "tiger stripe" cracks on the surface and more amorphous ice between these regions.[151] The crystalline ice near the tiger stripes could be explained by higher temperatures caused by geological activity that is the suspected cause of the cracks. The amorphous ice might be explained by flash freezing from cryovolcanism, rapid condensation of molecules from water geysers, or irradiation of high-energy particles from Saturn.[151] Similarly, one of one of the inner layers of Titan is believed to contain Ice VI.[175]
Medium-density amorphous ice may be present on Europa, as the experimental conditions of its formation are expected to occur there as well. It is possible that the MDA ice's unique property of releasing a large amount of heat energy after being released from compression could be responsible for 'ice quakes' within the thick ice layers.[22]
Planets[edit]
Because ice XI can theoretically form at low pressures at temperatures between 50–70 K – temperatures present in astrophysical environments of the outer solar system and within permanently shaded polar craters on the Moon and Mercury. Ice XI forms most easily around 70 K – paradoxically, it takes longer to form at lower temperatures. Extrapolating from experimental measurements, it is estimated to take ~50 years to form at 70 K and ~300 million years at 50 K.[176] It is theorized to be present in places like the upper atmospheres of Uranus and Neptune[98] and on Pluto and Charon.[176]
Ice VII may comprise the ocean floor of Europa as well as extrasolar planets (such as Awohali, and Enaiposha) that are largely made of water.[177][178]
Small domains of ice XI could exist in the atmospheres of Jupiter and Saturn as well.[98] The fact that small domains of ice XI can exist at temperatures up to 111 K has some scientists speculating that it may be fairly common in interstellar space, with small 'nucleation seeds' spreading through space and converting regular ice, much like the fabled ice-nine mentioned in Vonnegut's Cat's Cradle.[98][179] The possible roles of ice XI in interstellar space[176][180] and planet formation[181] have been the subject of several research papers. Until observational confirmation of ice XI in outer space is made, the presence of ice XI in space remains controversial owing to the aforementioned criticism raised by Iitaka.[182] The infrared absorption spectra of ice XI was studied in 2009 in preparation for searches for ice XI in space.[183]
It is theorized that the ice giant planets Uranus and Neptune hold a layer of superionic water.[184][117] [185][118] Machine learning and free-energy methods predict close-packed superionic phases to be stable over a wide temperature and pressure range, and a body-centred cubic superionic phase to be kinetically favoured, but stable over a small window of parameters.[186] On the other hand, there are also studies that suggest that other elements present inside the interiors of these planets, particularly carbon, may prevent the formation of superionic water.[187][188]
Notes[edit]
- ^ a b C0, C1, and C2 are all stable solid phases of a mixture of H2 and H2O molecules, formed at high pressures.[65][66] Although sometimes referred to as clathrate hydrates (or clathrates), they lack the cagelike structure generally found in clathrate hydrates, and are more properly referred to as filled ices.[65][66][67]
- ^ If kept at a temperature range between 110 and 120 K (−163 and −153 °C; −262 and −244 °F), after about two hours, the structure will have emptied itself of any detectable hydrogen molecules.[65][66]
- ^ One millibar is equivalent to 100 Pa (0.015 psi; 0.00099 atm).
References[edit]
- ^ La Placa, S. J.; Hamilton, W. C.; Kamb, B.; Prakash, A. (1972). "On a nearly proton ordered structure for ice IX". Journal of Chemical Physics. 58 (2): 567–580. Bibcode:1973JChPh..58..567L. doi:10.1063/1.1679238.
- ^ Klotz, S.; Besson, J. M.; Hamel, G.; Nelmes, R. J.; Loveday, J. S.; Marshall, W. G. (1999). "Metastable ice VII at low temperature and ambient pressure". Nature. 398 (6729): 681–684. Bibcode:1999Natur.398..681K. doi:10.1038/19480. S2CID 4382067.
- ^ Dutch, Stephen. "Ice Structure". University of Wisconsin Green Bay. Archived from the original on 16 October 2016. Retrieved 12 July 2017.
- ^ Bjerrum, N (11 April 1952). "Structure and Properties of Ice". Science. 115 (2989): 385–390. Bibcode:1952Sci...115..385B. doi:10.1126/science.115.2989.385. PMID 17741864.
- ^ a b Rottger, K.; Endriss, A.; Ihringer, J.; Doyle, S.; Kuhs, W. F. (1994). "Lattice Constants and Thermal Expansion of H2O and D2O Ice Ih Between 10 and 265 K". Acta Crystallogr. B50 (6): 644–648. doi:10.1107/S0108768194004933.
- ^ a b David T. W. Buckingham, J. J. Neumeier, S. H. Masunaga, and Yi-Kuo Yu (2018). "Thermal Expansion of Single-Crystal H2O and D2O Ice Ih". Physical Review Letters. 121 (18): 185505. Bibcode:2018PhRvL.121r5505B. doi:10.1103/PhysRevLett.121.185505. PMID 30444387.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ P. W. Bridgman (1912). "Water, in the Liquid and Five Solid Forms, under Pressure". Proceedings of the American Academy of Arts and Sciences. 47 (13): 441–558. doi:10.2307/20022754. JSTOR 20022754.
- ^ Velikov, V.; Borick, S; Angell, C. A. (2001). "Estimation of water-glass transition temperature based on hyperquenched glassy water experiments". Science. 294 (5550): 2335–8. Bibcode:2001Sci...294.2335V. doi:10.1126/science.1061757. PMID 11743196. S2CID 43859537.
- ^ Martelli, Fausto; Torquato, Salvatore; Giovambattista, Nicolas; Car, Roberto (2017-09-29). "Large-Scale Structure and Hyperuniformity of Amorphous Ices". Physical Review Letters. 119 (13): 136002. arXiv:1705.09961. Bibcode:2017PhRvL.119m6002M. doi:10.1103/PhysRevLett.119.136002. PMID 29341697. S2CID 44864111.
- ^ Martelli, Fausto; Leoni, Fabio; Sciortino, Francesco; Russo, John (2020-09-14). "Connection between liquid and non-crystalline solid phases in water". The Journal of Chemical Physics. 153 (10): 104503. Bibcode:2020JChPh.153j4503M. doi:10.1063/5.0018923. hdl:11573/1440448. ISSN 0021-9606. PMID 32933306. S2CID 221746507.
- ^ a b Conde, M.M.; Vega, C.; Tribello, G.A.; Slater, B. (2009). "The phase diagram of water at negative pressures: Virtual ices". The Journal of Chemical Physics. 131 (34510): 034510. Bibcode:2009JChPh.131c4510C. doi:10.1063/1.3182727. PMID 19624212.

- ^ Militzer, Burkhard; Wilson, Hugh F. (2 November 2010). "New Phases of Water Ice Predicted at Megabar Pressures". Physical Review Letters. 105 (19): 195701. arXiv:1009.4722. Bibcode:2010PhRvL.105s5701M. doi:10.1103/PhysRevLett.105.195701. PMID 21231184. S2CID 15761164.
- ^ MacMahon, J. M. (1970). "Ground-State Structures of Ice at High-Pressures". Physical Review B. 84 (22): 220104. arXiv:1106.1941. Bibcode:2011PhRvB..84v0104M. doi:10.1103/PhysRevB.84.220104. S2CID 117870442.
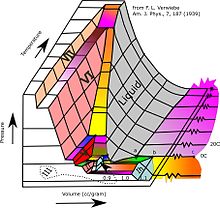
- ^ David, Carl (8 August 2016). "Verwiebe's '3-D' Ice phase diagram reworked". Chemistry Education Materials.
- ^ Wagner, Wolfgang; Saul, A.; Pruss, A. (May 1994). "International Equations for the Pressure Along the Melting and Along the Sublimation Curve of Ordinary Water Substance". Journal of Physical and Chemical Reference Data. 23 (3): 515–527. Bibcode:1994JPCRD..23..515W. doi:10.1063/1.555947.
- ^ Murphy, D. M. (2005). "Review of the vapour pressures of ice and supercooled water for atmospheric applications". Quarterly Journal of the Royal Meteorological Society. 131 (608): 1539–1565. Bibcode:2005QJRMS.131.1539M. doi:10.1256/qj.04.94. S2CID 122365938.
- ^ "SI base units". Bureau International des Poids et Mesures. Archived from the original on 16 July 2012. Retrieved 31 August 2012.
- ^ "Information for users about the proposed revision of the SI" (PDF). Bureau International des Poids et Mesures. Archived from the original (PDF) on 21 January 2018. Retrieved 6 January 2019.
- ^ Iglev, H.; Schmeisser, M.; Simeonidis, K.; Thaller, A.; Laubereau, A. (2006). "Ultrafast superheating and melting of bulk ice". Nature. 439 (7073): 183–186. Bibcode:2006Natur.439..183I. doi:10.1038/nature04415. PMID 16407948. S2CID 4404036.
- ^ Köster KW, Fuentes-Landete V, Raidt A, Seidl M, Gainaru C, Loerting T; et al. (2018). "Author Correction: Dynamics enhanced by HCl doping triggers 60% Pauling entropy release at the ice XII-XIV transition". Nat Commun. 9: 16189. Bibcode:2018NatCo...916189K. doi:10.1038/ncomms16189. PMC 6026910. PMID 29923547.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fuentes-Landete V; Köster KW; Böhmer R; Loerting T (2018). "Thermodynamic and kinetic isotope effects on the order-disorder transition of ice XIV to ice XII". Phys Chem Chem Phys. 20 (33): 21607–21616. Bibcode:2018PCCP...2021607F. doi:10.1039/c8cp03786h. PMID 30101255. S2CID 51969440.
- ^ a b c Rosu-Finsen, Alexander; Davies, Michael B.; Amon, Alfred; Wu, Han; Sella, Andrea; Michaelides, Angelos; Salzmann, Christoph G. (3 February 2023). "Medium-density amorphous ice". Science. 379 (6631): 474–478. Bibcode:2023Sci...379..474R. doi:10.1126/science.abq2105. ISSN 0036-8075. PMID 36730416. S2CID 256504172.
- ^ Bernal, J. D.; Fowler, R. H. (1 January 1933). "A Theory of Water and Ionic Solution, with Particular Reference to Hydrogen and Hydroxyl Ions". The Journal of Chemical Physics. 1 (8): 515. Bibcode:1933JChPh...1..515B. doi:10.1063/1.1749327.
- ^ Berg, Bernd A.; Muguruma, Chizuru; Okamoto, Yuko (2007-03-21). "Residual entropy of ordinary ice from multicanonical simulations". Physical Review B. 75 (9). arXiv:cond-mat/0609211. doi:10.1103/PhysRevB.75.092202. ISSN 1098-0121.
- ^ Pauling, Linus (1 December 1935). "The Structure and Entropy of Ice and of Other Crystals with Some Randomness of Atomic Arrangement". Journal of the American Chemical Society. 57 (12): 2680–2684. doi:10.1021/ja01315a102.
- ^ Petrenko, Victor F.; Whitworth, Robert W. (2002-01-17). "2. Ice Ih". Physics of Ice. Oxford University Press. doi:10.1093/acprof:oso/9780198518945.003.0002. ISBN 978-0-19-851894-5.
- ^ Nagle, J. F. (1966-08-01). "Lattice Statistics of Hydrogen Bonded Crystals. I. The Residual Entropy of Ice". Journal of Mathematical Physics. 7 (8): 1484–1491. doi:10.1063/1.1705058. ISSN 0022-2488.
- ^ Hollins, G. T. (December 1964). "Configurational statistics and the dielectric constant of ice". Proceedings of the Physical Society. 84 (6): 1001. doi:10.1088/0370-1328/84/6/318. ISSN 0370-1328.
- ^ Flatz, Christian; Hohenwarter, Stefan. "Neue kristalline Eisform aus Innsbruck". Universität Innsbruck (in German). Retrieved 2021-02-18.
- ^ Murray, B.J.; Bertram, A. K. (2006). "Formation and stability of cubic ice in water droplets". Phys. Chem. Chem. Phys. 8 (1): 186–192. Bibcode:2006PCCP....8..186M. doi:10.1039/b513480c. hdl:2429/33770. PMID 16482260.
- ^ Murray, B.J. (2008). "The Enhanced formation of cubic ice in aqueous organic acid droplets". Env. Res. Lett. 3 (2): 025008. Bibcode:2008ERL.....3b5008M. doi:10.1088/1748-9326/3/2/025008.
- ^ Dowell, L. G.; Rinfret, A. P. (December 1960). "Low-Temperature Forms of Ice as Studied by X-Ray Diffraction". Nature. 188 (4757): 1144–1148. Bibcode:1960Natur.188.1144D. doi:10.1038/1881144a0. ISSN 0028-0836. S2CID 4180631.
- ^ a b c d Jenniskens, Peter; Blake, David F. (1994). "Structural transitions in amorphous water ice and astrophysical implications". Science. 265 (5173): 753–6. Bibcode:1994Sci...265..753J. doi:10.1126/science.11539186. PMID 11539186.
- ^ Jenniskens P.; Blake D. F. (1996). "Crystallization of amorphous water ice in the solar system". Astrophysical Journal. 473 (2): 1104–13. Bibcode:1996ApJ...473.1104J. doi:10.1086/178220. PMID 11539415. S2CID 33622340.
- ^ Jenniskens P.; Banham S. F.; Blake D. F.; McCoustra M. R. (July 1997). "Liquid water in the domain of cubic crystalline ice Ic". Journal of Chemical Physics. 107 (4): 1232–41. Bibcode:1997JChPh.107.1232J. doi:10.1063/1.474468. PMID 11542399.
- ^ "Scientists made a new kind of ice that might exist on distant moons". Nature. 4 February 2023.
- ^ Sullivan, Will (3 February 2023). "Scientists Have Created a New Type of Ice - It looks like a white powder and has nearly the same density as liquid water". Smithsonian Magazine. Retrieved 4 February 2023.
- ^ a b c d Mishima O.; Calvert L. D.; Whalley E. (1984). "'Melting ice' I at 77 K and 10 kbar: a new method of making amorphous solids". Nature. 310 (5976): 393–395. Bibcode:1984Natur.310..393M. doi:10.1038/310393a0. S2CID 4265281.
- ^ a b Jenniskens P.; Blake D. F.; Wilson M. A.; Pohorille A. (1995). "High-density amorphous ice, the frost on insterstellar grains". Astrophysical Journal. 455: 389. Bibcode:1995ApJ...455..389J. doi:10.1086/176585. hdl:2060/19980018148. S2CID 122950585.
- ^ Mishima, O.; Calvert, L. D.; Whalley, E. (1985). "An apparently 1st-order transition between two amorphous phases of ice induced by pressure". Nature. 314 (6006): 76–78. Bibcode:1985Natur.314...76M. doi:10.1038/314076a0. S2CID 4241205.
- ^ O.Mishima (1996). "Relationship between melting and amorphization of ice". Nature. 384 (6609): 546–549. Bibcode:1996Natur.384..546M. doi:10.1038/384546a0. S2CID 4274283.
- ^ Loerting, Thomas; Salzmann, Christoph; Kohl, Ingrid; Mayer, Erwin; Hallbrucker, Andreas (2001). "A second distinct structural "state" of high-density amorphous ice at 77 K and 1 bar". Physical Chemistry Chemical Physics. 3 (24): 5355–5357. Bibcode:2001PCCP....3.5355L. doi:10.1039/b108676f. S2CID 59485355.
- ^ a b c d e f g Hobbs, Peter V. (May 6, 2010). Ice Physics. Oxford University Press. pp. 61–70. ISBN 9780199587711. Retrieved December 6, 2014.
- ^ a b Chaplin, Martin (October 18, 2014). "Ice-two structure". Water Structure and Science. London South Bank University. Retrieved December 6, 2014.
- ^ "Ice III (ice-three) structure". 2012-02-04. Archived from the original on 2012-02-04. Retrieved 2023-06-06.
- ^ Chaplin, Martin (10 April 2012). "Ice-four (Ice IV)". Water Structure and Science. London South Bank University. Archived from the original on 12 August 2011. Retrieved 27 May 2022.
- ^ a b Chaplin, Martin (10 April 2012). "Ice-five (Ice V)". Water Structure and Science. London South Bank University. Archived from the original on 12 October 2003. Retrieved 30 July 2012.
- ^ Drost-Hansen, W. (1969-11-14). "The Structure and Properties of Water. D. Eisenberg and W. Kauzmann. Oxford University Press, New York, 1969. xiv + 300 pp., illus. Cloth, $10; paper, $4.50". Science. 166 (3907): 861. doi:10.1126/science.166.3907.861. ISSN 0036-8075.
- ^ Chaplin, Martin (20 December 2019). "Ice-five (Ice V)". Archived from the original on 2020-08-13. Retrieved 5 June 2021.
- ^ Kamb, B.; Prakash, A.; Knobler, C. (1967). "Structure of ice. V". Acta Crystallographica. 22 (5): 706. doi:10.1107/S0365110X67001409.
- ^ Water, in the Liquid and Five Solid Forms, under Pressure P.W. Bridgman (1912), www.jstor.org, retrieved 3 October 2019
- ^ Reports: Structure of Ice VI science.sciencemag.org, B. Kamb, 8 October 1965.
- ^ Chaplin, Martin (10 April 2012). "Ice-six (Ice VI)". Water Structure and Science. London South Bank University. Archived from the original on 23 September 2012. Retrieved 30 July 2012.
- ^ a b c Grande, Zachary M.; et al. (2022). "Pressure-driven symmetry transitions in dense H2O ice". APS Physics. 105 (10): 104109. Bibcode:2022PhRvB.105j4109G. doi:10.1103/PhysRevB.105.104109. S2CID 247530544.
- ^ Chaplin, Martin (July 1, 2007). "Ice-eight structure". Water Structure and Science. Retrieved January 2, 2008.
- ^ La Placa, Sam J.; Hamilton, Walter C.; Kamb, Barclay; Prakash, Anand (1973-01-15). "On a nearly proton‐ordered structure for ice IX". The Journal of Chemical Physics. 58 (2): 567–580. doi:10.1063/1.1679238. ISSN 0021-9606.
- ^ a b Chaplin, Martin (10 April 2012). "Ice-seven (Ice VII)". Water Structure and Science. London South Bank University. Archived from the original on 2 November 2011. Retrieved 30 July 2012.
- ^ Chaplin, Martin (17 February 2017). "Ice-eleven (ice XI)". Water Structure and Science. London South Bank University. Archived from the original on 23 March 2017. Retrieved 11 March 2017.
- ^ C. Lobban, J.L. Finney and W.F. Kuhs, The structure of a new phase of ice, Nature 391, 268–270, 1998
- ^ a b Salzmann, Christoph G.; Radaelli, Paolo G.; Hallbrucker, Andreas; Mayer, Erwin; Finney, John L. (24 March 2006). "The Preparation and Structures of Hydrogen Ordered Phases of Ice". Science. 311 (5768): 1758–1761. Bibcode:2006Sci...311.1758S. doi:10.1126/science.1123896. PMID 16556840. S2CID 44522271.
- ^ a b c d e f g Chaplin, Martin (10 April 2012). "Ice-twelve (Ice XII)". Water Structure and Science. London South Bank University. Archived from the original on 2 November 2011. Retrieved 30 July 2012.
- ^ Salzmann CG, Radaelli PG, Hallbrucker A, Mayer E, Finney JL (2006). "The preparation and structures of hydrogen ordered phases of ice". Science. 311 (5768): 1758–61. Bibcode:2006Sci...311.1758S. doi:10.1126/science.1123896. PMID 16556840. S2CID 44522271.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sanders, Laura (11 September 2009). "A Very Special Snowball". Science News. Archived from the original on 14 September 2009. Retrieved 11 September 2009.
- ^ a b Chaplin, Martin. "Ice-seventeen (Ice XVII)". Archived from the original on 2022-09-11. Retrieved 2022-09-11.
{{cite web}}: CS1 maint: bot: original URL status unknown (link)[self-published source?] - ^ a b c d e f g h i j k l m n o p del Rosso, Leonardo; Celli, Milva; Ulivi, Lorenzo (7 November 2016). "New porous water ice metastable at atmospheric pressure obtained by emptying a hydrogen-filled ice". Nature Communications. 7 (1): 13394. arXiv:1607.07617. Bibcode:2016NatCo...713394D. doi:10.1038/ncomms13394. PMC 5103070. PMID 27819265.
- ^ a b c d e f g h Chaplin, Martin. "Ice-seventeen (Ice XVII)". Archived from the original on 2022-09-11. Retrieved 2022-09-11.
{{cite web}}: CS1 maint: bot: original URL status unknown (link)[self-published source?] - ^ a b c d Liu, Yuan; Huang, Yingying; Zhu, Chongqin; Li, Hui; Zhao, Jijun; Wang, Lu; Ojamäe, Lars; Francisco, Joseph S.; Zeng, Xiao Cheng (25 June 2019). "An ultralow-density porous ice with the largest internal cavity identified in the water phase diagram". Proceedings of the National Academy of Sciences. 116 (26): 12684–12691. Bibcode:2019PNAS..11612684L. doi:10.1073/pnas.1900739116. PMC 6600908. PMID 31182582.
- ^ a b Falenty, Andrzej; Hansen, Thomas C.; Kuhs, Werner F. (December 2014). "Formation and properties of ice XVI obtained by emptying a type sII clathrate hydrate". Nature. 516 (7530): 231–233. Bibcode:2014Natur.516..231F. doi:10.1038/nature14014. PMID 25503235. S2CID 4464711.
- ^ Falenty, A.; Hansen, T. C.; Kuhs, W. F. (2014). "Formation and properties of ice XVI obtained by emptying a type sII clathrate hydrate". Nature. 516 (7530): 231–233. Bibcode:2014Natur.516..231F. doi:10.1038/nature14014. PMID 25503235. S2CID 4464711.

- ^ Jacobson, Liam C.; Hujo, Waldemar; Molinero, Valeria (2009). "Thermodynamic Stability and Growth of Guest-Free Clathrate Hydrates: A Low-Density Crystal Phase of Water". Journal of Physical Chemistry B. 113 (30): 10298–10307. doi:10.1021/jp903439a. PMID 19585976.

- ^ Algara-Siller, G.; Lehtinen, O.; Wang, F. C.; Nair, R. R.; Kaiser, U.; Wu, H. A.; Geim, A. K.; Grigorieva, I. V. (2015-03-26). "Square ice in graphene nanocapillaries". Nature. 519 (7544): 443–445. arXiv:1412.7498. Bibcode:2015Natur.519..443A. doi:10.1038/nature14295. PMID 25810206. S2CID 4462633.
- ^ a b "Sandwiching water between graphene makes square ice crystals at room temperature". ZME Science. 2015-03-27. Retrieved 2018-05-02.
- ^ a b Cite error: The named reference
xvii.discoverywas invoked but never defined (see the help page). - ^ Chaplin, Martin. "Ice-seventeen (Ice XVII)". Archived from the original on 11 September 2022. Retrieved 12 September 2022.
{{cite web}}: CS1 maint: bot: original URL status unknown (link)[self-published source?] - ^ a b c Gasser, TM; Thoeny, AV; Plaga, LJ; Köster, KW; Etter, M; Böhmer, R; et al. (2018). "Experiments indicating a second hydrogen ordered phase of ice VI". Chem Sci. 9 (18): 4224–4234. doi:10.1039/c8sc00135a. PMC 5942039. PMID 29780552.
- ^ a b c Metcalfe, Tom (9 March 2021). "Exotic crystals of 'ice 19' discovered". Live Science.
- ^ a b Yamane R, Komatsu K, Gouchi J, Uwatoko Y, Machida S, Hattori T, Kagi H; et al. (2021). "Experimental evidence for the existence of a second partially-ordered phase of ice VI". Nat Commun. 12 (1): 1129. Bibcode:2021NatCo..12.1129Y. doi:10.1038/s41467-021-21351-9. PMC 7893076. PMID 33602936.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shephard, J. J., Slater, B., Harvey, P., Hart, M., Bull, C. L., Bramwell, S. T., Salzmann, C. G. (2018), "Doping-induced disappearance of ice II from water's phase diagram", Nature Physics, 14 (6), Springer Science and Business Media LLC: 569–572, Bibcode:2018NatPh..14..569S, doi:10.1038/s41567-018-0094-z, S2CID 54544973
- ^ Nakamura, T., Matsumoto, M., Yagasaki, T., Tanaka, H. (2015), "Thermodynamic Stability of Ice II and Its Hydrogen-Disordered Counterpart: Role of Zero-Point Energy", The Journal of Physical Chemistry B, 120 (8), American Chemical Society (ACS): 1843–1848, doi:10.1021/acs.jpcb.5b09544, PMID 26595233
- ^ Engelhardt, H., Kamb, B. (1981), "Structure of ice IV, a metastable high‐pressure phase", The Journal of Chemical Physics, 75 (12), AIP Publishing: 5887–5899, doi:10.1063/1.442040
- ^ Shephard, J. J., Ling, S., Sosso, G. C., Michaelides, A., Slater, B., Salzmann, C. G. (2017), "Is High-Density Amorphous Ice Simply a "Derailed" State along the Ice I to Ice IV Pathway?", The Journal of Physical Chemistry Letters, 8 (7), American Chemical Society (ACS): 1645–1650, arXiv:1701.05398, doi:10.1021/acs.jpclett.7b00492, PMID 28323429, S2CID 13662778
- ^ Engelhardt, H., Whalley, E. (1979), "The infrared spectrum of ice IV in the range 4000–400 cm−1", The Journal of Chemical Physics, 71 (10), AIP Publishing: 4050–4051, doi:10.1063/1.438173
- ^ Salzmann, C. G., Kohl, I., Loerting, T., Mayer, E., Hallbrucker, A. (2003), "Raman Spectroscopic Study on Hydrogen Bonding in Recovered Ice IV", The Journal of Physical Chemistry B, 107 (12), American Chemical Society (ACS): 2802–2807, doi:10.1021/jp021534k
- ^ Colin Lobban (1998), Neutron diffraction studies of ices, University College London, ProQuest 1752797359
- ^ Klotz, S., Hamel, G., Loveday, J. S., Nelmes, R. J., Guthrie, M. (2003), "Recrystallisation of HDA ice under pressure by in-situ neutron diffraction to 3.9 GPa", Zeitschrift für Kristallographie - Crystalline Materials, 218 (2), Walter de Gruyter GmbH: 117–122, doi:10.1524/zkri.218.2.117.20669, S2CID 96109290
- ^ Bridgman, P. W. (1935), "The Pressure‐Volume‐Temperature Relations of the Liquid, and the Phase Diagram of Heavy Water", The Journal of Chemical Physics, 3 (10), AIP Publishing: 597–605, doi:10.1063/1.1749561
- ^ Evans, L. F. (1967), "Selective Nucleation of the High‐Pressure Ices", Journal of Applied Physics, 38 (12), AIP Publishing: 4930–4932, doi:10.1063/1.1709255
- ^ Salzmann, C. G., Loerting, T., Kohl, I., Mayer, E., Hallbrucker, A. (2002), "Pure Ice IV from High-Density Amorphous Ice", The Journal of Physical Chemistry B, 106 (22), American Chemical Society (ACS): 5587–5590, doi:10.1021/jp014391v
- ^ Salzmann, CG; Radaelli, PG; Slater, B; Finney, JL (2011), "The polymorphism of ice: five unresolved questions.", Phys Chem Chem Phys, 13 (41): 18468–80, doi:10.1039/c1cp21712g, PMID 21946782
- ^ Rosu-Finsen, A., Salzmann, C. G. (2022), "Is pressure the key to hydrogen ordering ice IV?", Chemical Physics Letters, 789, Elsevier BV: 139325, doi:10.1016/j.cplett.2021.139325, S2CID 245597764
- ^ Fan, Xiaofeng; Bing, Dan; Zhang, Jingyun; Shen, Zexiang; Kuo, Jer-Lai (1 October 2010). "Predicting the hydrogen bond ordered structures of ice Ih, II, III, VI and ice VII: DFT methods with localized based set" (PDF). Computational Materials Science. 49 (4): S170–S175. doi:10.1016/j.commatsci.2010.04.004. Archived from the original (PDF) on 14 July 2014. Retrieved 24 April 2012.
- ^ Dengel, O.; Eckener, U.; Plitz, H.; Riehl, N. (1 May 1964). "Ferroelectric behavior of ice". Physics Letters. 9 (4): 291–292. Bibcode:1964PhL.....9..291D. doi:10.1016/0031-9163(64)90366-X.
- ^ Kawada, Shuji (1 May 1972). "Dielectric Dispersion and Phase Transition of KOH Doped Ice". Journal of the Physical Society of Japan. 32 (5): 1442. Bibcode:1972JPSJ...32.1442K. doi:10.1143/JPSJ.32.1442.
- ^ Tajima, Yoshimitsu; Matsuo, Takasuke; Suga, Hiroshi (1984). "Calorimetric study of phase transition in hexagonal ice doped with alkali hydroxides". Journal of Physics and Chemistry of Solids. 45 (11–12): 1135–1144. Bibcode:1984JPCS...45.1135T. doi:10.1016/0022-3697(84)90008-8.
- ^ Matsuo, Takasuke; Tajima, Yoshimitsu; Suga, Hiroshi (1986). "Calorimetric study of a phase transition in D2O ice Ih doped with KOD: Ice XI". Journal of Physics and Chemistry of Solids. 47 (2): 165–173. Bibcode:1986JPCS...47..165M. doi:10.1016/0022-3697(86)90126-5.
- ^ Castro Neto, A.; Pujol, P.; Fradkin, E. (2006). "Ice: A strongly correlated proton system". Physical Review B. 74 (2): 024302. arXiv:cond-mat/0511092. Bibcode:2006PhRvB..74b4302C. doi:10.1103/PhysRevB.74.024302. S2CID 102581583.
- ^ Arakawa, Masashi; Kagi, Hiroyuki; Fukazawa, Hiroshi (2010). "Annealing effects on hydrogen ordering in KOD-doped ice observed using neutron diffraction". Journal of Molecular Structure. 972 (1–3): 111–114. Bibcode:2010JMoSt.972..111A. doi:10.1016/j.molstruc.2010.02.016.
- ^ a b c d Arakawa, Masashi; Kagi, Hiroyuki; Fernandez-Baca, Jaime A.; Chakoumakos, Bryan C.; Fukazawa, Hiroshi (17 August 2011). "The existence of memory effect on hydrogen ordering in ice: The effect makes ice attractive". Geophysical Research Letters. 38 (16): n/a. Bibcode:2011GeoRL..3816101A. doi:10.1029/2011GL048217.
- ^ K. Abe, Y. Ootake and T. Shigenari, Raman scattering study of proton ordered ice XI single crystal, in Physics and Chemistry of Ice, ed. W. Kuhs (Royal Society of Chemistry, Cambridge, 2007) pp 101–108
- ^ Abe, K.; Shigenari, T. (2011). "Raman spectra of proton ordered phase XI of ICE I. Translational vibrations below 350 cm-1, J". The Journal of Chemical Physics. 134 (10): 104506. Bibcode:2011JChPh.134j4506A. doi:10.1063/1.3551620. PMID 21405174.
- ^ Raza, Zamaan; Alfè, Dario (28 Nov 2011). "Proton ordering in cubic ice and hexagonal ice; a potential new ice phase--XIc". Physical Chemistry Chemical Physics. 13 (44): 19788–95. Bibcode:2011PCCP...1319788R. doi:10.1039/c1cp22506e. PMID 22009223. S2CID 31673433.
- ^ Bramwell, Steven T. (21 January 1999). "Ferroelectric ice". Nature. 397 (6716): 212–213. Bibcode:1999Natur.397..212B. doi:10.1038/16594. S2CID 204990667.
- ^ Iedema, M. J.; Dresser, M. J.; Doering, D. L.; Rowland, J. B.; Hess, W. P.; Tsekouras, A. A.; Cowin, J. P. (1 November 1998). "Ferroelectricity in Water Ice". The Journal of Physical Chemistry B. 102 (46): 9203–9214. doi:10.1021/jp982549e. S2CID 97894870.
- ^ Su, Xingcai; Lianos, L.; Shen, Y.; Somorjai, Gabor (1998). "Surface-Induced Ferroelectric Ice on Pt(111)". Physical Review Letters. 80 (7): 1533–1536. Bibcode:1998PhRvL..80.1533S. doi:10.1103/PhysRevLett.80.1533. S2CID 121266617.
- ^ Zhao, H.-X.; Kong, X.-J.; Li, H.; Jin, Y.-C.; Long, L.-S.; Zeng, X. C.; Huang, R.-B.; Zheng, L.-S. (14 February 2011). "Transition from one-dimensional water to ferroelectric ice within a supramolecular architecture". Proceedings of the National Academy of Sciences. 108 (9): 3481–3486. Bibcode:2011PNAS..108.3481Z. doi:10.1073/pnas.1010310108. PMC 3048133. PMID 21321232.
- ^ Knight, Chris; Singer, Sherwin J. (2005-10-19). "Prediction of a Phase Transition to a Hydrogen Bond Ordered Form of Ice VI". The Journal of Physical Chemistry B. 109 (44). American Chemical Society (ACS): 21040–21046. doi:10.1021/jp0540609. ISSN 1520-6106.
- ^ Sanders, Laura (11 September 2009). "Super-Dense Frozen Water Breaks Final Ice Frontier". Wired. Condé Nast. Retrieved 13 September 2009.
- ^ Salzmann, Christoph G.; Radaelli, Paolo G.; Mayer, Erwin; Finney, John L. (2009). "Ice XV: A New Thermodynamically Stable Phase of Ice". Physical Review Letters. 103 (10): 105701. arXiv:0906.2489. Bibcode:2009PhRvL.103j5701S. doi:10.1103/PhysRevLett.103.105701. PMID 19792330. S2CID 13999983.
- ^ a b c del Rosso, Leonardo; Celli, Milva; Grazzi, Francesco; Catti, Michele; Hansen, Thomas C.; Fortes, A. Dominic; Ulivi, Lorenzo (June 2020). "Cubic ice Ic without stacking defects obtained from ice XVII". Nature Materials. 19 (6): 663–668. arXiv:1907.02915. Bibcode:2020NatMa..19..663D. doi:10.1038/s41563-020-0606-y. PMID 32015533. S2CID 195820566.
- ^ Chaplin, Martin. "Stacking disordered ice; Ice Isd". Archived from the original on 2022-09-11. Retrieved 2022-09-11.
{{cite web}}: CS1 maint: bot: original URL status unknown (link)[self-published source?] - ^ Komatsu, Kazuki; Machida, Shinichi; Noritake, Fumiya; Hattori, Takanori; Sano-Furukawa, Asami; Yamane, Ryo; Yamashita, Keishiro; Kagi, Hiroyuki (3 February 2020). "Ice Ic without stacking disorder by evacuating hydrogen from hydrogen hydrate". Nature Communications. 11 (1): 464. arXiv:1909.03400. Bibcode:2020NatCo..11..464K. doi:10.1038/s41467-020-14346-5. PMC 6997176. PMID 32015342.
- ^ Demontis, P.; et al. (1988). "New high-pressure phases of ice" (PDF). Phys. Rev. Lett. 60 (22): 2284–2287. doi:10.1103/PhysRevLett.60.2284. PMID 10038311.
- ^ Weird water lurking inside giant planets, New Scientist,01 September 2010, Magazine issue 2776.
- ^ a b c Millot, Marius; Coppari, Federica; Rygg, J. Ryan; Correa Barrios, Antonio; Hamel, Sebastien; Swift, Damian C.; Eggert, Jon H. (8 May 2019). "Nanosecond X-ray diffraction of shock-compressed superionic water ice". Nature. 569 (7755): 251–255. doi:10.1038/s41586-019-1114-6. OSTI 1568026. PMID 31068720. S2CID 256768272.
- ^ Goncharov, Alexander F.; et al. (2005). "Dynamic Ionization of Water under Extreme Conditions" (PDF). Phys. Rev. Lett. 94 (12): 125508. doi:10.1103/PhysRevLett.94.125508. PMID 15903935.
- ^ a b c Millot, Marius; et al. (5 February 2018). "Experimental evidence for superionic water ice using shock compression". Nature Physics. 14 (3): 297–302. Bibcode:2018NatPh..14..297M. doi:10.1038/s41567-017-0017-4. OSTI 1542614. S2CID 256703104.
- ^ a b Marris, Emma (22 March 2005). "Giant planets may host superionic water". Nature. doi:10.1038/news050321-4.
- ^ a b c Zyga, Lisa (25 April 2013). "New phase of water could dominate the interiors of Uranus and Neptune". Phys.org.
- ^ a b Sokol, Joshua (2019-05-12). "A Bizarre Form of Water May Exist All Over the Universe". Wired. ISSN 1059-1028. Retrieved 2019-05-13.
- ^ a b Chang, Kenneth (2018-02-05). "New Form of Water, Both Liquid and Solid, Is 'Really Strange'". The New York Times. ISSN 0362-4331. Retrieved 2018-02-13.
- ^ Rosu-Finsen, A; Salzmann, CG (2019). "Origin of the low-temperature endotherm of acid-doped ice VI: new hydrogen-ordered phase of ice or deep glassy states?". Chem Sci. 10 (2): 515–523. doi:10.1039/c8sc03647k. PMC 6334492. PMID 30713649.
- ^ Thoeny AV; Gasser TM; Loerting T (2019). "Distinguishing ice β-XV from deep glassy ice VI: Raman spectroscopy". Phys Chem Chem Phys. 21 (28): 15452–15462. Bibcode:2019PCCP...2115452T. doi:10.1039/c9cp02147g. PMID 31257365. S2CID 195764029.
- ^ Rosu-Finsen A, Amon A, Armstrong J, Fernandez-Alonso F, Salzmann CG (2020). "Deep-Glassy Ice VI Revealed with a Combination of Neutron Spectroscopy and Diffraction". J Phys Chem Lett. 11 (3): 1106–1111. doi:10.1021/acs.jpclett.0c00125. PMC 7008458. PMID 31972078.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gasser TM, Thoeny AV, Fortes AD, Loerting T (2021). "Structural characterization of ice XIX as the second polymorph related to ice VI". Nat Commun. 12 (1): 1128. Bibcode:2021NatCo..12.1128G. doi:10.1038/s41467-021-21161-z. PMC 7892819. PMID 33602946.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Salzmann CG, Loveday JS, Rosu-Finsen A, Bull CL (2021). "Structure and nature of ice XIX". Nat Commun. 12 (1): 3162. Bibcode:2021NatCo..12.3162S. doi:10.1038/s41467-021-23399-z. PMC 8155070. PMID 34039987.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Norman Anderson. "The Many Phases of Ice" (PDF) (Physics 511 paper). Iowa State University. Archived from the original (PDF) on 7 October 2009.
- ^ Atkins, Peter; de Paula, Julio (2010). Physical chemistry (9th ed.). New York: W. H. Freeman and Co. p. 144. ISBN 978-1429218122.
- ^ Murray, Benjamin J.; Knopf, Daniel A.; Bertram, Allan K. (2005). "The formation of cubic ice under conditions relevant to Earth's atmosphere". Nature. 434 (7030): 202–205. Bibcode:2005Natur.434..202M. doi:10.1038/nature03403. PMID 15758996. S2CID 4427815.
- ^ Whalley, E. (1981). "Scheiner's Halo: Evidence for Ice Ic in the Atmosphere". Science. 211 (4480): 389–390. Bibcode:1981Sci...211..389W. doi:10.1126/science.211.4480.389. PMID 17748273.
- ^ Murray, Benjamin J.; Salzmann, Christoph G.; Heymsfield, Andrew J.; Dobbie, Steven; Neely, Ryan R.; Cox, Christopher J. (2015). "Trigonal Ice Crystals in Earth's Atmosphere" (PDF). Bulletin of the American Meteorological Society. 96 (9): 1519–1531. Bibcode:2015BAMS...96.1519M. doi:10.1175/BAMS-D-13-00128.1.
- ^ Chaplin, Martin (15 September 2019). "Stacking disordered ice; Ice Isd". Water Structure and Science. London South Bank University. Archived from the original on 22 Oct 2020. Retrieved 3 December 2019.
- ^ Malkin, Tamsin L.; Murray, Benjamin J.; Salzmann, Christoph G.; Molinero, Valeria; Pickering, Steven J.; Whale, Thomas F. (2015). "Stacking disorder in ice I". Physical Chemistry Chemical Physics. 17 (1): 60–76. doi:10.1039/c4cp02893g. PMID 25380218.
- ^ Kuhs, W. F.; Sippel, C.; Falenty, A.; Hansen, T. C. (2012). "Extent and relevance of stacking disorder in "ice Ic"". Proceedings of the National Academy of Sciences of the United States of America. 109 (52): 21259–21264. Bibcode:2012PNAS..10921259K. doi:10.1073/pnas.1210331110. PMC 3535660. PMID 23236184.
- ^ Salzmann, Christoph G.; Murray, Benjamin J. (June 2020). "Ice goes fully cubic". Nature Materials. 19 (6): 586–587. Bibcode:2020NatMa..19..586S. doi:10.1038/s41563-020-0696-6. PMID 32461682. S2CID 218913209.
- ^ Komatsu K, Machida S, Noritake F, Hattori T, Sano-Furukawa A, Yamane R; et al. (2020). "Ice Ic without stacking disorder by evacuating hydrogen from hydrogen hydrate". Nat Commun. 11 (1): 464. arXiv:1909.03400. Bibcode:2020NatCo..11..464K. doi:10.1038/s41467-020-14346-5. PMC 6997176. PMID 32015342.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Del Rosso L, Celli M, Grazzi F, Catti M, Hansen TC, Fortes AD; et al. (2020). "Cubic ice Ic without stacking defects obtained from ice XVII". Nat Mater. 19 (6): 663–668. arXiv:1907.02915. Bibcode:2020NatMa..19..663D. doi:10.1038/s41563-020-0606-y. PMID 32015533. S2CID 195820566.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lübken, F.-J.; Lautenbach, J.; Höffner, J.; Rapp, M.; Zecha, M. (March 2009). "First continuous temperature measurements within polar mesosphere summer echoes". Journal of Atmospheric and Solar-Terrestrial Physics. 71 (3–4): 453–463. Bibcode:2009JASTP..71..453L. doi:10.1016/j.jastp.2008.06.001.
- ^ Murray, Benjamin J.; Jensen, Eric J. (January 2010). "Homogeneous nucleation of amorphous solid water particles in the upper mesosphere". Journal of Atmospheric and Solar-Terrestrial Physics. 72 (1): 51–61. Bibcode:2010JASTP..72...51M. doi:10.1016/j.jastp.2009.10.007.
- ^ Murray, Benjamin J.; Malkin, Tamsin L.; Salzmann, Christoph G. (May 2015). "The crystal structure of ice under mesospheric conditions". Journal of Atmospheric and Solar-Terrestrial Physics. 127: 78–82. Bibcode:2015JASTP.127...78M. doi:10.1016/j.jastp.2014.12.005.
- ^ O. Tschauner; S Huang; E. Greenberg; V.B. Prakapenka; C. Ma; G.R. Rossman; A.H. Shen; D. Zhang; M. Newville; A. Lanzirotti; K. Tait (2018). "Ice-VII inclusions in diamonds: Evidence for aqueous fluid in Earth's deep mantle". Science. 359 (6380): 1136–1139. Bibcode:2018Sci...359.1136T. doi:10.1126/science.aao3030. PMID 29590042. S2CID 206662912.
- ^ Sid Perkins (2018-03-08). "Pockets of water may lay deep below Earth's surface". Science. Archived from the original on March 8, 2018. Retrieved March 8, 2018.
- ^ Netburn, Deborah. "What scientists found trapped in a diamond: a type of ice not known on Earth". Los Angeles Times. Archived from the original on 12 March 2018. Retrieved 12 March 2018.
- ^ Fukazawa, Hiroshi; Mae, Shinji; Ikeda, Susumu; Watanabe, Okitsugu (1998). "Proton ordering in Antarctic ice observed by Raman and neutron scattering". Chemical Physics Letters. 294 (6): 554–558. Bibcode:1998CPL...294..554F. doi:10.1016/S0009-2614(98)00908-7.
- ^ Fortes, A. D.; Wood, I. G.; Grigoriev, D.; Alfredsson, M.; Kipfstuhl, S.; Knight, K. S.; Smith, R. I. (1 January 2004). "No evidence for large-scale proton ordering in Antarctic ice from powder neutron diffraction". The Journal of Chemical Physics. 120 (24): 11376–9. Bibcode:2004JChPh.12011376F. doi:10.1063/1.1765099. PMID 15268170. Archived from the original on 29 July 2012. Retrieved 22 April 2012.
- ^ Furić, K.; Volovšek, V. (2010). "Water ice at low temperatures and pressures; new Raman results". J. Mol. Structure. 976 (1–3): 174–180. Bibcode:2010JMoSt.976..174F. doi:10.1016/j.molstruc.2010.03.024.
- ^ Yen, Fei; Chi, Zhenhua (16 Apr 2015). "Proton ordering dynamics of H2O ice". Physical Chemistry Chemical Physics. 17 (19): 12458–12461. arXiv:1503.01830. Bibcode:2015PCCP...1712458Y. doi:10.1039/C5CP01529D. PMID 25912948. S2CID 7736338.
- ^ Dubochet, J.; Adrian, M.; Chang, J. .J; Homo, J. C.; Lepault, J-; McDowall, A. W.; Schultz, P. (1988). "Cryo-electron microscopy of vitrified specimens" (PDF). Quarterly Reviews of Biophysics. 21 (2): 129–228. doi:10.1017/S0033583500004297. PMID 3043536. S2CID 2741633.
- ^ a b c d e f g h Del Rosso, Leonardo; Celli, Milva; Ulivi, Lorenzo (June 2017). "Ice XVII as a Novel Material for Hydrogen Storage". Challenges. 8 (1): 3. doi:10.3390/challe8010003.
- ^ Chang, Kenneth (9 December 2004). "Astronomers Contemplate Icy Volcanoes in Far Places". The New York Times. Archived from the original on 9 May 2015. Retrieved 30 July 2012.
- ^ Debennetti, Pablo G.; Stanley, H. Eugene (2003). "Supercooled and Glassy Water" (PDF). Physics Today. 56 (6): 40–46. Bibcode:2003PhT....56f..40D. doi:10.1063/1.1595053. Retrieved 19 September 2012.
- ^ a b c Newman, Sarah F.; Buratti, B. J.; Brown, R. H.; Jaumann, R.; Bauer, J.; Momary, T. (2008). "Photometric and spectral analysis of the distribution of crystalline and amorphous ices on Enceladus as seen by Cassini" (PDF). Icarus. 193 (2): 397–406. Bibcode:2008Icar..193..397N. doi:10.1016/j.icarus.2007.04.019. hdl:1721.1/114323.
- ^ Grundy, W. M.; Schmitt, B. (1998). "The temperature-dependent near-infrared absorption spectrum of hexagonal <formula>H2O ice". Journal of Geophysical Research. 103 (E11): 25809. Bibcode:1998JGR...10325809G. doi:10.1029/98je00738.
- ^ Hagen, W.; ielens, A.G.G.M.; Greenberg, J. M. (1981). "The Infrared Spectra of Amorphous Solid Water and Ice Between 10 and 140 K". Chemical Physics. 56 (3): 367–379. Bibcode:1981CP.....56..367H. doi:10.1016/0301-0104(81)80158-9.
- ^ a b c Moore, Marla H.; Hudson, Reggie L. (1992). "Far-infrared spectral studies of phase changes in water ice induced by proton irradiation". Astrophysical Journal. 401: 353. Bibcode:1992ApJ...401..353M. doi:10.1086/172065.
- ^ Smith, R. G.; Robinson, G.; Hyland, A. R.; Carpenter, G. L. (1994). "Molecular ices as temperature indicators for interstellar dust: the 44- and 62-μm lattice features of H2O ice". Monthly Notices of the Royal Astronomical Society. 271 (2): 481–489. Bibcode:1994MNRAS.271..481S. doi:10.1093/mnras/271.2.481.
- ^ Seki, J.; Hasegawa, H. (1983). "The heterogeneous condensation of interstellar ice grains". Astrophysics and Space Science. 94 (1): 177–189. Bibcode:1983Ap&SS..94..177S. doi:10.1007/BF00651770. S2CID 121008219.
- ^ Murray, B. J.; Jensen, E. J. (2010). "Homogeneous nucleation of amorphous solid water particles in the upper mesosphere". J. Atmos. Sol.-Terr. Phys. 72 (1): 51–61. Bibcode:2010JASTP..72...51M. doi:10.1016/j.jastp.2009.10.007.
- ^ a b Jenniskens; Blake; Kouchi (1998). Solar System Ices. Dordrecht Kluwer Academic Publishers. pp. 139–155.
- ^ a b c Kouchi, A.; Yamamoto, T.; Kozasa, T.; Kuroda, T.; Greenberg, J. M. (1994). "Conditions for condensation and preservation of amorphous ice and crystallinity of astrophysical ices" (PDF). Astronomy and Astrophysics. 290: 1009. Bibcode:1994A&A...290.1009K. Archived (PDF) from the original on 22 March 2020.
- ^ Kouchi, Akira; Kuroda, Toshio (1990). "Amorphization of cubic ice by ultraviolet irradiation". Nature. 344 (6262): 134–135. Bibcode:1990Natur.344..134K. doi:10.1038/344134a0. S2CID 4306842.
- ^ Jenniskens, P.; Blake, D. F.; Wilson, M. A.; Pohorille, A. (1995). "High-Density Amorphous Ice, the Frost on Interstellar Grains". Astrophysical Journal. 401: 389. Bibcode:1995ApJ...455..389J. doi:10.1086/176585. hdl:2060/19980018148. S2CID 122950585.
- ^ Omont, Alain; Forveille, Thierry; Moseley, S. Harvey; Glaccum, William J.; Harvey, Paul M.; Likkel, Lauren Jones; Loewenstein, Robert F.; Lisse, Casey M. (May 20, 1990), "Observations of 40-70 micron bands of ice in IRAS 09371 + 1212 and other stars", Astrophysical Journal Letters, 355: L27–L30, Bibcode:1990ApJ...355L..27O, doi:10.1086/185730, ISSN 0004-637X
- ^ Patashnick, et.al., Nature Vol.250, No. 5464, July 1974, pp. 313-314.
- ^ Meech, K. J.; Pittichová, J.; Bar-Nun, A.; Notesco, G.; Laufer, D.; Hainaut, O. R.; Lowry, S. C.; Yeomans, D. K.; Pitts, M. (2009). "Activity of comets at large heliocentric distances pre-perihelion". Icarus. 201 (2): 719–739. Bibcode:2009Icar..201..719M. doi:10.1016/j.icarus.2008.12.045.
- ^ Tancredi, G.; Rickman, H.; Greenberg, J. M. (1994). "Thermochemistry of cometary nuclei 1: The Jupiter family case". Astronomy and Astrophysics. 286: 659. Bibcode:1994A&A...286..659T.
- ^ Gronkowski, P. (2007). "The search for a cometary outbursts mechanism: a comparison of various theories". Astronomische Nachrichten. 328 (2): 126–136. Bibcode:2007AN....328..126G. doi:10.1002/asna.200510657.
- ^ Hosek, Matthew W. Jr.; Blaauw, Rhiannon C.; Cooke, William J.; Suggs, Robert M. (2013). "Outburst Dust Production of Comet 29P/Schwassmann-Wachmann 1". The Astronomical Journal. 145 (5): 122. Bibcode:2013AJ....145..122H. doi:10.1088/0004-6256/145/5/122.
- ^ Jewitt, David C.; Luu, Jane X. (2001). "Colors and Spectra of Kuiper Belt Objects". The Astronomical Journal. 122 (4): 2099–2114. arXiv:astro-ph/0107277. Bibcode:2001AJ....122.2099J. doi:10.1086/323304. S2CID 35561353.
- ^ Brown, Robert H.; Cruikshank, Dale P.; Pendleton, Yvonne (1999). "Water Ice on Kuiper Belt Object 1996 TO_66". The Astrophysical Journal. 519 (1): L101. Bibcode:1999ApJ...519L.101B. doi:10.1086/312098.
- ^ Fornasier, S.; Dotto, E.; Barucci, M. A.; Barbieri, C. (2004). "Water ice on the surface of the large TNO 2004 DW". Astronomy and Astrophysics. 422 (2): L43. Bibcode:2004A&A...422L..43F. doi:10.1051/0004-6361:20048004.
- ^ Jewitt, David C.; Luu, Jane (2004). "Crystalline water ice on the Kuiper belt object (50000) Quaoar". Nature. 432 (7018): 731–3. Bibcode:2004Natur.432..731J. doi:10.1038/nature03111. PMID 15592406. S2CID 4334385.
- ^ Spencer, John R.; Tamppari, Leslie K.; Martin, Terry Z.; Travis, Larry D. (1999). "Temperatures on Europa from Galileo Photopolarimeter-Radiometer: Nighttime Thermal Anomalies". Science. 284 (5419): 1514–1516. Bibcode:1999Sci...284.1514S. doi:10.1126/science.284.5419.1514. PMID 10348736.
- ^ a b Hansen, Gary B.; McCord, Thomas B. (2004). "Amorphous and crystalline ice on the Galilean satellites: A balance between thermal and radiolytic processes". Journal of Geophysical Research. 109 (E1): E01012. Bibcode:2004JGRE..109.1012H. doi:10.1029/2003JE002149. S2CID 140162310.
- ^ Showman, A. (1997). "Coupled Orbital and Thermal Evolution of Ganymede" (PDF). Icarus. 129 (2): 367–383. Bibcode:1997Icar..129..367S. doi:10.1006/icar.1997.5778.
- ^ "Titan: Facts - NASA Science". science.nasa.gov. Retrieved 25 April 2024.
- ^ a b c McKinnon, W. B.; Hofmeister, A.M. (August 2005). "Ice XI on Pluto and Charon?". Bulletin of the American Astronomical Society. 37 (49.02). Division for Planetary Sciences Meeting, American Astronomical Society: 732. Bibcode:2005DPS....37.4902M.
- ^ University of Liège (2007, May 16). Astronomers Detect Shadow Of Water World In Front Of Nearby Star. ScienceDaily. Retrieved Jan. 3, 2010, from "Astronomers Detect Shadow of Water World in Front of Nearby Star". Archived from the original on 2017-08-21. Retrieved 2018-04-22.
- ^ David A. Aguilar (2009-12-16). "Astronomers Find Super-Earth Using Amateur, Off-the-Shelf Technology". Harvard-Smithsonian Center for Astrophysics. Archived from the original on April 7, 2012. Retrieved January 23, 2010.
- ^ Grossman, Lisa (25 August 2011). "Electric ice a shock to the solar system". New Scientist. Retrieved 7 April 2012.
- ^ Fukazawa, H.; Hoshikawa, A.; Ishii, Y.; Chakoumakos, B. C.; Fernandez-Baca, J. A. (20 November 2006). "Existence of Ferroelectric Ice in the Universe". The Astrophysical Journal. 652 (1): L57–L60. Bibcode:2006ApJ...652L..57F. doi:10.1086/510017.
- ^ Iedema, M. J.; Dresser, M. J.; Doering, D. L.; Rowland, J. B.; Hess, W. P.; Tsekouras, A. A.; Cowin, J. P. (1998). "Ferroelectricity in Water Ice". The Journal of Physical Chemistry B. 102 (46). American Chemical Society (ACS): 9203–9214. doi:10.1021/jp982549e. ISSN 1520-6106.
- ^ Iitaka, Toshiaki (13 July 2010). "Stability of ferroelectric ice". arXiv:1007.1792 [cond-mat.mtrl-sci].
- ^ Arakawa, M.; Kagi, H.; Fukazawa, H. (1 October 2009). "Laboratory Measurements of Infrared Absorption Spectra of Hydrogen-Ordered Ice: a Step to the Exploration of Ice XI in Space". The Astrophysical Journal Supplement Series. 184 (2): 361–365. Bibcode:2009ApJS..184..361A. doi:10.1088/0067-0049/184/2/361.
- ^ Chang, Kenneth (5 February 2018). "Newly Discovered Form of Water Ice Is 'Really Strange' – Long theorized to be found in the mantles of Uranus and Neptune, the confirmation of the existence of superionic ice could lead to the development of new materials". The New York Times. Retrieved 5 February 2018.
- ^ Charlie Osolin. "Public Affairs Office: Recreating the Bizarre State of Water Found on Giant Planets". Llnl.gov. Retrieved 24 December 2010.
- ^ Cheng, Bingqing; Bethkenhagen, Mandy; Pickard, Chris J.; Hamel, Sebastien (2021). "Phase behaviours of superionic water at planetary conditions". Nature Physics. 17 (11): 1228–1232. arXiv:2103.09035. doi:10.1038/s41567-021-01334-9. S2CID 232240463.
- ^ Chau, Ricky; Hamel, Sebastien; Nellis, William J. (2011). "Chemical processes in the deep interior of Uranus". Nat. Commun. 2. Article number: 203. doi:10.1038/ncomms1198. PMID 21343921.
- ^ Wang, Yanchao (29 November 2011). "High pressure partially ionic phase of water ice". Nature Communications. 2: 563. doi:10.1038/ncomms1566. PMID 22127059.
Further reading[edit]
- Ice phases (www.idc-online.com)
- Fletcher, N. H. (2009-06-04). The Chemical Physics of Ice. ISBN 9780521112307.
- Petrenko, Victor F.; Whitworth, Robert W. (1999-08-19). Physics of Ice. ISBN 9780191581342.
- Chaplin, Martin (2007-11-11). "Hexagonal ice structure". Water Structure and Science. Retrieved 2008-01-02.
- London South Bank University Report
- Physik des Eises (PDF in German, iktp.tu-dresden.de)
External links[edit]
- Hunsberger, Maren (September 21, 2018). "A New State of Water Reveals a Hidden Ocean in Earth's Mantle". Seeker. Archived from the original on 2021-12-21 – via YouTube.
- Woo, Marcus (July 11, 2018). "The Hunt for Earth's Deep Hidden Oceans". Quanta Magazine.
- Discussion of amorphous ice at LSBU's website.
- Glass transition in hyperquenched water from Nature (requires registration)
- Glassy Water from Science, on phase diagrams of water (requires registration)
- AIP accounting discovery of VHDA
- HDA in space
- Computerized illustrations of molecular structure of HDA