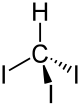
Iodoform
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Triiodomethane
| |||
| Other names
Iodoform, Methyl triiodide, Carbon triiodide, TIM
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ECHA InfoCard | 100.000.795 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CHI3 | |||
| Molar mass | 393.73 g/mol | ||
| Appearance | Yellow crystals | ||
| Density | 4.008 g/cm3 | ||
| Melting point | 123 °C | ||
| Boiling point | 217 °C (expl.) | ||
| 0.1 g/L at 20 °C (slightly soluble in glycerol and petroleum ether; moderately soluble in chloroform and acetic acid, highly soluble in benzene, ethanol (78 g/L at 25 °C), acetone (120 g/L at 25 °C) and ether (136 g/L at 25 °C)) | |||
| log P | 3.83 | ||
Henry's law
constant (kH) |
0.34 mol.kg-1.bar-1 | ||
| Structure | |||
| Hexagonal | |||
| Tetrahedral | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 204 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Iodoform is the organoiodine compound with the formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating odor (in older chemistry texts, the smell is sometimes referred to as the smell of hospitals) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant.
Synthesis and reactions
Iodoform was first prepared by Georges Serrulas in 1822 and its molecular formula was identified by Jean-Baptiste Dumas in 1834. It is synthesized in the haloform reaction by the reaction of iodine and sodium hydroxide with any one of these four kinds of organic compounds: (i) a methyl ketone: CH3COR, where R is an organic side chain, acetaldehyde (CH3CHO), ethanol (CH3CH2OH), and certain secondary alcohols (CH3CHROH, where R is an alkyl or aryl group).
The reaction of iodine and base with methyl ketones is so reliable, that the "iodoform test" (the appearance of a yellow precipitate) is used to probe the presence of a methyl ketone.
Some reagents (e.g. Hydrogen iodide) convert iodoform to diiodomethane. Also conversion to carbon dioxide is possible: Iodoform reacts with aqueous silver nitrate to produce carbon monoxide, which is oxidized by mixture of sulfuric acid and iodine pentaoxide.
Applications
The compound finds small scale use as a disinfectant.[1] Around the beginning of the 20th century it was used in medicine as a healing and antiseptic dressing for wounds and sores, although this use is now superseded by superior antiseptics. It is the active ingredient in many ear powders for dogs and cats, to prevent infection and facilitate removal of ear hair, along with zinc oxide and boric acid.
References
- ^ Phyllis A. Lyday "Iodine and Iodine Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- Merck Index, 12 Edition, 5054.
See also
External links
- NIOSH Pocket Guide to Chemical Hazards. "#0343". National Institute for Occupational Safety and Health (NIOSH).
- MSDS at Oxford University
- MSDS at Science Lab
- A Method for the Specific Conversion of Iodoform to Carbon Dioxide
- Article at 1911 Encyclopaedia Britannica
- Preparation