Iron(III) pyrophosphate
| |
| Names | |
|---|---|
| Other names
Ferric pyrophosphate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.030.160 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
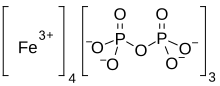
| Fe4(P2O7)3 | |
| Molar mass | 745.224 (anhydrate) 907.348 (nonahydrate) |
| Appearance | yellow solid (nonahydrate)[1] |
| insoluble | |
| Pharmacology | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iron(III) pyrophosphate is an inorganic chemical compound with the formula Fe4(P2O7)3.
Synthesis[edit]
Anhydrous iron(III) pyrophosphate can be prepared by heating the mixture of iron(III) metaphosphate and iron(III) phosphate under oxygen with the stoichiometric ratio 1:3. The reactants can be prepared by reacting iron(III) nitrate nonahydrate with phosphoric acid.[5]
It can be also prepared via the following reaction:[6]
- 3 Na4P2O7(aq) + 4 FeCl3(aq) → Fe4(P2O7)3(s) + 12 NaCl(aq)
References[edit]
- ^ W.M.Haynes. CRC Handbook of Chemistry and Physics (97th edition). New York: CRC Press, 2016. pp 4-68
- ^ "Summary Basis of Decision (SBD) for Triferic Avnu". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ "Triferic Avnu- ferric pyrophosphate citrate solution". DailyMed. 15 September 2020. Retrieved 29 May 2022.
- ^ Elbouaanani, L.K; Malaman, B; Gérardin, R; Ijjaali, M (2002). "Crystal Structure Refinement and Magnetic Properties of Fe4(P2O7)3 Studied by Neutron Diffraction and Mössbauer Techniques". Journal of Solid State Chemistry. 163 (2). Elsevier BV: 412–420. Bibcode:2002JSSCh.163..412E. doi:10.1006/jssc.2001.9415. ISSN 0022-4596.
- ^ Rossi L, Velikov KP, Philipse AP (May 2014). "Colloidal iron(III) pyrophosphate particles". Food Chem. 151: 243–7. doi:10.1016/j.foodchem.2013.11.050. PMID 24423528.
External links[edit]
- "Ferric pyrophosphate nonahydrate". Drug Information Portal. U.S. National Library of Medicine.
- "Ferric pyrophosphate citrate". Drug Information Portal. U.S. National Library of Medicine.
