Iron(II) oxalate

| |

| |
| Names | |
|---|---|
| IUPAC name
Iron(II) oxalate
| |
| Other names
Iron oxalate
Ferrous oxalate | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.007.472 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| FeC2O4 (anhydrous) FeC2O4 · 2 H2O (dihydrate) | |
| Molar mass | 143.86 g/mol (anhydrous) 179.89 g/mol (dihydrate) |
| Appearance | yellow powder |
| Odor | odorless |
| Density | 2.28 g/cm3 |
| Melting point | dihydrate: 150–160 °C (302–320 °F; 423–433 K) (decomposes) |
| dihydrate: 0.097 g/100ml (25 °C)[1] | |
| Hazards | |
| GHS labelling: | |
 [2] [2]
| |
| Warning | |
| H302, H312[2] | |
| P280[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferrous oxalate (iron(II) oxalate) are inorganic compound with the formula FeC2O4(H2O)x where x is 0 or 2. These are orange compounds, poorly soluble in water.
Structure and reactions[edit]
Like other iron oxalates, ferrous oxalates feature octahedral Fe centers. The dihydrate FeC2O4(H2O)x is a coordination polymer, consisting of chains of oxalate-bridged ferrous centers, each with two aquo ligands.[3]
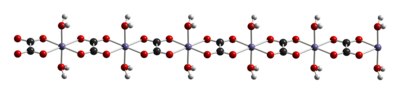
When heated to 120 °C, the dihydrate dehydrates, and the anhydrous ferrous oxalate decomposes near 190 °C.[4] The products of thermal decomposition is a mixture of iron oxides and pyrophoric iron metal, as well as released carbon dioxide, carbon monoxide, and water.[5]
Ferrous oxalates are precursors to iron phosphates, which are of value in batteries.[6]
Natural occurrence[edit]
Anhydrous iron(II) oxalate is unknown among minerals as of 2020. However, the dihydrate is known as humboldtine.[7][8] A related, though much more complex mineral is stepanovite,
Na[Mg(H2O)6] [Fe3+(C2O4)3]·3H2O - an example of trioxalatoferrate(III).[9][8]
See also[edit]
References[edit]
- ^ "Iron(II) oxalate dihydrate".
- ^ a b c Sigma-Aldrich Co., Iron(II) oxalate dihydrate. Retrieved on 2014-05-03.
- ^ Echigo, Takuya; Kimata, Mitsuyoshi (2008). "Single-crystal X-ray diffraction and spectroscopic studies on humboldtine and lindbergite: weak Jahn–Teller effect of Fe2+ ion". Physics and Chemistry of Minerals. 35 (8): 467–475. Bibcode:2008PCM....35..467E. doi:10.1007/s00269-008-0241-7. S2CID 98739882.
- ^ Mu, Jacob; Perlmutter, D.D. (1981). "Thermal decomposition of carbonates, carboxylates, oxalates, acetates, formates, and hydroxides". Thermochimica Acta. 49 (2–3): 207–218. doi:10.1016/0040-6031(81)80175-x.
- ^ Hermanek, Martin; Zboril, Radek; Mashlan, Miroslav; Machala, Libor; Schneeweiss, Oldrich (2006). "Thermal Behaviour of Iron(II) Oxalate Dihydrate in the Atmosphere of Its Conversion Gases". J. Mater. Chem. 16 (13): 1273–1280. doi:10.1039/b514565a.
- ^ Ellis, B. L.; Makahnouk, W. R. M.; Makimura, Y.; Toghill, K.; Nazar, L. F. (2007). "A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries". Nature Materials. 6 (10): 749–753. Bibcode:2007NatMa...6..749E. doi:10.1038/nmat2007. PMID 17828278.
- ^ "Humboldtine".
- ^ a b "List of Minerals". 21 March 2011.
- ^ "Stepanovite".
