Metalorganic vapour-phase epitaxy
It has been suggested that Vapour phase decomposition be merged into this article. (Discuss) Proposed since April 2012. |
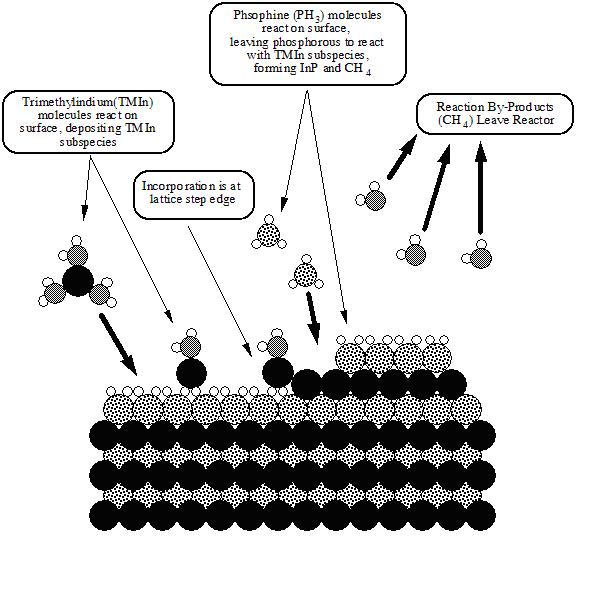
Metalorganic vapour phase epitaxy (MOVPE), also known as organometallic vapour phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method of epitaxial growth of materials, especially compound semiconductors, from the surface reaction of organic compounds or metalorganics and hydrides containing the required chemical elements. For example, indium phosphide could be grown in a reactor on a substrate by introducing Trimethylindium ((CH3)3In) and phosphine (PH3). Formation of the epitaxial layer occurs by final pyrolysis of the constituent chemicals at the substrate surface. In contrast to molecular beam epitaxy (MBE) the growth of crystals is by chemical reaction and not physical deposition. This takes place not in a vacuum, but from the gas phase at moderate pressures (2 to 100 kPa). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics.
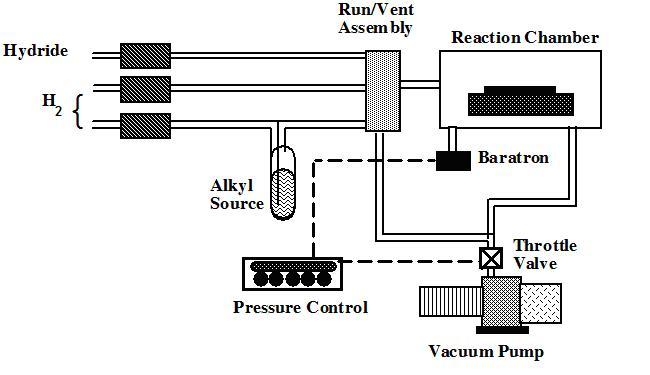
Reactor components
- A reactor is a chamber made of a material that does not react with the chemicals being used. It must also withstand high temperatures. This chamber is composed by reactor walls, liner, a susceptor, gas injection units, and temperature control units. Usually, the reactor walls are made from stainless steel or quartz. To prevent overheating, cooling water must be flowing through the channels within the reactor walls. Ceramic or special glasses, such as quartz, are often used as the liner in the reactor chamber between the reactor wall and the susceptor. A substrate sits on a susceptor which is at a controlled temperature. The susceptor is made from a material resistant to the metalorganic compounds used; graphite is sometimes used. For growing nitrides and related materials, a special coating on the graphite susceptor is necessary to prevent corrosion by ammonia (NH3) gas. In cold-wall CVD, only the susceptor is heated, so gases do not react before they reach the hot wafer surface. In hot-wall CVD, the entire chamber is heated. This may be necessary for some gases to be pre-cracked before reaching the wafer surface to allow them to stick to the wafer.
- Gas inlet and switching system. Gas is introduced via devices known as 'bubblers'. In a bubbler a carrier gas (usually nitrogen or hydrogen) is bubbled through the metalorganic liquid, which picks up some metalorganic vapour and transports it to the reactor. The amount of metalorganic vapour transported depends on the rate of carrier gas flow and the bubbler temperature, and is usually controlled automatically and most accurately by using a Piezocon type vapour control system. Allowance must be made for saturated vapours.
- Pressure maintenance system
- Gas Exhaust and cleaning System. Toxic waste products must be converted to liquid or solid wastes for recycling (preferably) or disposal. Ideally processes will be designed to minimize the production of waste products.
Organometallic precursors
- Aluminium
- Trimethylaluminium (TMA or TMAl), Liquid
- Triethylaluminium (TEA or TEAl), Liquid
- Gallium
- Trimethylgallium (TMG or TMGa), Liquid
- Triethylgallium (TEG or TEGa), Liquid
- Indium
- Trimethylindium (TMI or TMIn), Solid
- Triethylindium (TEI or TEIn), Liquid
- Di-isopropylmethylindium (DIPMeIn), Liquid
- Ethyldimethylindium (EDMIn), Liquid
- Germanium
- Isobutylgermane (IBGe), Liquid
- Dimethylamino germanium trichloride (DiMAGeC), Liquid
- Tetramethylgermane (TMGe), Liquid
- Tetraethylgermane (TEGe), Liquid
- Nitrogen
- Phenyl hydrazine, Liquid
- Dimethylhydrazine (DMHy), Liquid
- Tertiarybutylamine (TBAm), Liquid
- Ammonia NH3, Gas
- Phosphorus
- Phosphine PH3, Gas
- Tertiarybutyl phosphine (TBP), Liquid
- Bisphosphinoethane (BPE), Liquid
- Arsenic
- Arsine AsH3, Gas
- Tertiarybutyl arsine (TBAs), Liquid
- Monoethyl arsine (MEAs), Liquid
- Trimethyl arsine (TMAs), Liquid
- Antimony
- Trimethyl antimony (TMSb), Liquid
- Triethyl antimony (TESb), Liquid
- Tri-isopropyl antimony (TIPSb), Liquid
- Stibine SbH3, Gas
- Cadmium
- Dimethyl cadmium (DMCd), Liquid
- Diethyl cadmium (DECd), Liquid
- Methyl Allyl Cadmium (MACd), Liquid
- Tellurium
- Dimethyl telluride (DMTe), Liquid
- Diethyl telluride (DETe), Liquid
- Di-isopropyl telluride (DIPTe), Liquid
- Selenium
- Dimethyl selenide (DMSe), Liquid
- Diethyl selenide (DESe), Liquid
- Di-isopropyl selenide (DIPSe), Liquid
- Zinc
- Dimethylzinc (DMZ), Liquid
- Diethylzinc (DEZ), Liquid
Semiconductors grown by MOVPE
III-V semiconductors
- AlGaAs
- AlGaInP
- AlGaN
- AlGaP
- GaAsP
- GaAs
- GaN
- GaP
- InAlAs
- InAlP
- InSb
- InGaN
- GaInAlAs
- GaInAlN
- GaInAsN
- GaInAsP
- GaInAs
- GaInP
- InN
- InP
- InAs
II-VI semiconductors
- Zinc selenide (ZnSe)
- HgCdTe
- ZnO
- Zinc sulfide (ZnS)
IV Semiconductors
IV-V-VI Semiconductors
Environment, Health and Safety
As MOVPE has become well-established production technology, there are equally growing concerns associated with its bearing on personnel and community safety, environmental impact and maximum quantities of hazardous materials (such as gases and metalorganics) permissible in the device fabrication operations. The safety as well as responsible environmental care have become major factors of paramount importance in the MOVPE-based crystal growth of compound semiconductors.
